View/Open
advertisement

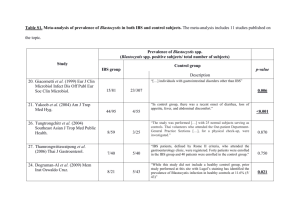
Sensitivity testing in Irritable Bowel Syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity? short title: TRPV1 in visceral hypersensitivity & IBS Sander JM van Wanrooij1,3, Mira M Wouters1,3, Lukas Van Oudenhove1, Winde Vanbrabant1, Stéphanie Mondelaers1, Patrick Kollmann2, Florian Kreutz2, Michael Schemann2, Guy Boeckxstaens1 1 Translational Research Center for Gastrointestinal Disorders, Leuven University, Leuven, Belgium 2 Department of Human Biology, TU Munchen, Freising, Germany 3 Both authors contributed equally Corresponding author: Prof. dr. G. Boeckxstaens Translational Research Center for Gastrointestinal Disorders, Leuven University Herestraat 49 bus 701 3000 Leuven, Belgium Tel (32) 16 33 08 37 Fax (32) 16 Email: guy.boeckxstaens@med.kuleuven.be Word count: 5056 1 Abstract: Sensitivity testing in Irritable Bowel Syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity Objectives: Abnormal pain perception or visceral hypersensitivity (VH) is considered to be an important mechanism underlying symptoms in a subgroup of irritable bowel syndrome (IBS) patients. Increased TRPV1 expression in rectal biopsies of IBS patients suggests a potentially important role for this nociceptor in the pathophysiology of IBS. Evidence underscoring the involvement of TRPV1 in visceral perception in IBS however is lacking. The objective of this study was to evaluate the role of TRPV1 in visceral hypersensitivity to rectal distension and clinical symptoms in patients with IBS. Methods: 48 IBS patients and 25 healthy volunteers (HVs) were invited to undergo subsequent assessment of sensitivity to rectal distensions and rectal capsaicin applications. Visceral sensitivity was evaluated by rectal distension at 3, 9 and 21 mmHg above minimal distension pressure. Capsaicin was applied to the rectal mucosa (0.01%, 0.1% or solvent only in random order). Visceral sensations (urge to defecate, pain, burning and warmth sensation) were scored on a 100mm visual analogue scale (VAS). TRPV1 expression in rectal biopsies was determined by immunohistochemistry and Real-Time PCR. Results: 23 IBS patients (48%) were hypersensitive to rectal distensions (VH-IBS). A concentration dependent increase of urge and pain perception was present in HVs and IBS patients during 2 capsaicin 0.01% and 0.1% applications. VH-IBS patients experienced a significantly increased perception of pain, but not urge, during capsaicin applications compared to normosensitive patients (ns-IBS) and HVs. Increased pain perception was significantly associated with anxiety and visceral hypersensitivity, symptoms scores of abdominal pain, loose stools and stool frequency. Anxiety experienced during the experimental procedure was enhanced in VH-IBS patients, but not ns-IBS or HVs. However, rectal TRPV1 expression was similar in VHIBS, ns-IBS and HVs on both mRNA and protein expression level. TRPV1 expression levels did not correlate with pain perception to capsaicin or clinical symptoms in IBS patients or the subgroups. Conclusions: IBS patients with VH to rectal distension reveal increased pain perception to rectal application of capsaicin, as well as an increased anxiety response. No evidence for TRPV1 upregulation could be demonstrated. As both visceral hypersensitivity and anxiety are independently associated with increased pain perception to rectal capsaicin application, our data suggest that both peripheral and central factors are involved, with increased receptor sensitivity as a speculative possibility. 3 Study highlights: 1) WHAT IS CURRENT KNOWLEDGE: Visceral hypersensitivity (VH) is an important mechanism underlying irritable bowel syndrome (IBS). Expression of the nociceptor TRPV1 is increased in rectal biopsies of IBS patients and in animal models of VH. 2) WHAT IS NEW HERE IBS patients with VH to rectal distension have an increased pain response to the TRPV1-agonist capsaicin. TRPV1 mRNA or protein expression is not increased in the rectal mucosa of IBS patients, including VH. Anxiety levels and visceral hypersensitivity are independently associated with increased pain responses to capsaicin 4 Introduction The irritable Bowel Syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by recurrent episodes of abdominal pain and discomfort associated with changes in bowel frequency and consistency. IBS is a symptom based diagnosis and belongs to the most frequently diagnosed diseases among gastroenterologist worldwide, with an estimated prevalence of 20% in the western world (1). Due to the chronic nature of the disease, its morbidity is high leading to reduced quality of life and a high burden on health care costs. IBS is a multifactorial disorder with several pathophysiological factors involved, including visceral hypersensitivity (VH), abnormal gastrointestinal motility, dietary factors, psychological and genetic factors, aberrant neuro-immune interactions and increased mucosal permeability (2;3). VH, defined as increased perception of both physiological and noxious stimuli, is considered an important therapeutic target and is present in 21-94% of IBS patients (4-11). Psychological factors such as anxiety and depression are associated with VH and alterations in central processing (12;13), indicating that central mechanisms such as anticipation or arousal-anxiety driven failure of descending pain inhibition may contribute. On the other hand, sensitization of afferent nerves has repeatedly been shown to represent an important mechanism leading to abnormal perception in preclinical models (14;15), mainly resulting from sensitization and upregulation of nociceptors (16-19), including the transient receptor potential cation channel subfamily V member 1 (TRPV1). This nociceptor is activated by capsaicin, the active component of red pepper, heat (above 42˚C), acidosis (pH < 6), and endovanilloids. Importantly, its activation threshold is significantly lowered under inflammatory conditions by mediators such as prostaglandins, bradykinin, anandamide, ATP and lipoxygenase products (12-HPETE, 15-HPETE)(20;21). The potentially important role for TRPV1 in VH pathogenesis is confirmed in animal models. VH to colorectal distension (CRD) in rats, induced by neonatal acetic acid treatment or water avoidance stress, can be reversed by TRPV1 antagonist administration (18;22). A similar effect of TRPV1 5 antagonist administration was reported in two studies using a model of trinitrobenzene sulfonic acid (TNBS) colitis induced VH to CRD (17;23). In line, increased expression of TRPV1 in dorsal root ganglia (DRG) has been demonstrated in chemical-induced VH models (17;23). Finally, sensitivity to CRD is significantly decreased in TRPV1 knockout mice, an effect mimicking TRPV1-antagonist administration, thereby further underscoring the involvement of TRPV1 upregulation in preclinical models of VH (24). Not only in rodents, but also in patients with IBS, evidence has been reported indicating increased TRPV1 immunoreactivity in rectal biopsies (25). Median numbers of immunoreactive fibers in rectosigmoid biopsies from 23 IBS patients showed a 3.5 fold increase correlating with abdominal pain scores in a comparison with 22 healthy controls. Furthermore, these rectosigmoid biopsies revealed signs of low-grade inflammation and neuronal sprouting. The authors concluded that the increased TRPV1 nerve fibers may contribute to VH and pain in IBS, and provide a novel therapeutic target (25). However, as no functional data were reported on visceral sensitivity, it remains unclear to what extent TRPV1 expression is preferentially increased in patients with visceral hypersensitivity. Moreover, if TRPV1 expression is indeed increased and involved in abnormal visceral perception, hypersensitive patients should have an increased pain response during rectal stimulation with capsaicin, a TRPV1 agonist. Previously, Drewes et al. reported a consistent pain response evoked by application of capsaicin to ileal and colonic mucosa in healthy volunteers. Based on these observations, the authors proposed mucosal application of capsaicin as an acceptable model to study visceral pain (26;27). In the present study, we used a comparable test to evaluate the hypothesis that TRPV1 is upregulated in visceral hypersensitive IBS patients leading to increased perception of rectal capsaicin applications. To this end, 48 IBS patients and 25 healthy subjects underwent assessment of visceral sensitivity using rectal distensions whereas expression of TRPV1 in rectal biopsies and symptoms to rectal application were assessed by quantitative PCR and immunohistochemistry. 6 Materials and Methods Study subjects Healthy subjects were recruited by public advertisement; none of the healthy subjects had symptoms or a history of gastrointestinal disease, previous gastrointestinal surgery (except uncomplicated appendectomy) or were taking any gastrointestinal medication. Patients were recruited from the outpatient clinic of the University Hospital Leuven and had to fulfill the Rome III criteria for IBS (28). Patients were further sub-classified according to the ROME III criteria based on the predominant stool pattern: diarrhea predominant (IBS-D, constipation predominant (IBS-C), mixed diarrhea and constipation predominant periods (IBS-M) or unspecified if no other sub-classification criteria were fulfilled (IBS-U). In addition to careful history taking, all patients underwent a minimal work-up to exclude organic disease. This included a physical examination, biochemistry (including thyroid stimulating hormone levels and antibodies to anti-tissue transglutaminase), stool analysis and sigmoidoscopy or colonoscopy. Patients had to be free of relevant concomitant disease, such as diabetes, and active treatment of psychiatric disorders. Concomitant medication likely to interfere with gastrointestinal tract function or visceral perception (other than fibers or bulking agents), was discontinued at least 3 days before the study, or at least 7 days in case of antidepressants. Informed consent was obtained from each participant and the study protocol has been approved by the Ethics Committee of the University Hospital Leuven (October 2009). Symptom questionnaires Gastrointestinal symptoms, including abdominal bloating, flatulence, frequency of bowel movements, stool consistency, urgency and the feeling of incomplete evacuation were scored using the validated Gastrointestinal Symptom Rating Scale (GSRS) (29). The intensity of symptoms is scored on a 7-graded Likert scale with descriptive anchors (0 = no symptoms at all; 1 = minimal symptoms; 2 = mild symptoms; 3 = moderate symptoms; 4 = rather serious symptoms; 5 = serious symptoms and 6 = very severe symptoms). A 5-point score was used to evaluate abdominal pain. Patients were also 7 requested to answer the following question: ‘Please consider how much abdominal pain you experienced in the past 4 weeks’. Possible answers were: 1 = none; 2 = mild; 3 = moderate; 4 = severe and 5 = very severe (4). Barostat studies To assess visceral sensitivity, symptoms evoked by rectal distension were evaluated. To this end, an electronic barostat, automatically correcting for the compressibility of air (Synetics Visceral Stimulator, Stockholm, Sweden) was connected to a polyethylene barostat bag (500 mL, with a maximal length of 13 cm). The bag was tightly wrapped on the distal end of a double lumen polyvinyl tube to connect it to the barostat device (Salem Sump tube 14 Ch.; Sherwood Medical, Petit rechain, Belgium). Before the study, subjects received a tap water enema, followed by a 20-min period of rest. The barostat bag was lubricated and positioned into the rectum. Subjects were studied in the left lateral decubitus position. After a 10-min adaptation period, minimal distending pressure (MDP) was determined. We defined MDP as the minimum pressure at which the corresponding intrabag volume was at least 30 mL. The distension protocol consisted of a series of 3 phasic, ascending isobaric distensions, of 3, 9 and 21 mmHg increment above MDP respectively. These distension pressures correspond with the thresholds for first sensation, first urge and first discomfort in IBS patients, as reported by us earlier (4;5). The inflation rate was 38 mL/s and each distension step lasted 2 min, separated by 1-min intervals at baseline (MDP). Sensations were scored at the end of each distension step. A 100mm Visual Analogue Scale (VAS) with verbal descriptors (0 = no sensation; 20 = first sensation; 40 = first sense of urge; 60 = normal urge to defecate; 80 = severe urge to defecate and 100 = discomfort/pain) was used to score evoked sensations. The barostat bag was instantaneously deflated if a subject reported discomfort or pain. Capsaicin studies After the series of three rectal distensions, a 10 min period of recovery was included. Thereafter, a proctoscope was introduced until 5 cm behind the anal canal to assess the sensitivity to capsaicin. 8 Each subject received three applications in random order: capsaicin in a low (0.01%) and high (0.1%) concentration and a sham application (solvent only). Capsaicin was prepared according a previously described method (26). Capsaicin was gently applied to the rectal mucosa with a cotton swab during anorectal proctoscopy. Swabs were applied at 5 to 10 cm behind the anal canal for one minute. The surface of the applications was one square centimeter with a distance of at least one centimeter between different applications sites. Four sensations were scored on a 100mm Visual Analogue Scale (100mm VAS): warmth, burning, urge to defecate and pain (Fig. 1). TRPV1 expression analysis During anorectal proctoscopy, rectal biopsy specimens were collected from 22 healthy volunteers (mean age: 29±2 years; 9 male, 13 female) and 42 IBS patients (mean age: 34±2; 16male, 26 female)(Table 2). Biopsies were transferred in RNALater (Qiagen, the Netherlands) and stored at −80°C until extraction of total RNA by RNeasy Minikit (Qiagen). Total RNA was reverse transcribed into first strand cDNA by qScript cDNA Supermix (Quanta Science, Gaithersburg, MD, USA) following the manufacturers protocol. Real-time PCR was performed in a final reaction volume of 20 µl on a LightCycler® 480 Real-Time PCR System (Roche Applied Science, Belgium) using SYBRGreen gene expression assays (Lightcycler 480 SYBRGreen I Mastermix, Roche Diagnostics, Belgium) for betaactin (5’-GACAGGATGCAGAAGGAGATTACT, 3’-TGATCCACATCTGCTGGAAGGT), UCHL1 (5’GGCCACCTCTATGAACTTGATGGAC, 3’-AGCGGACTTCTCCTTGCTCACG), TRPV1 (5’- CCCCGATAGCTCCTACAACA, 3’-GCAGCAGGATGATGAAGACA and TRPV4 (5’GCGAGGTCATTACGCTCTTC, 3’-TAGAGGGCTGCTGAGACGAT) according to the manufacturer’s instructions. All samples were amplified in triplicate reactions. The relative expression of the target gene was calculated relative to the β-actin reference mRNA(30). For immunohistochemical expression of TRPV1, biopsies were fixed overnight in 10% neutral buffered formalin (10% formaldehyde (obtained from a 37% solution (Fisher scientific, Landsmeer, The Netherlands), 10% phosphate concentrate (H2PO4 x H2O and Na2HPO4 x 7H2O, Sigma-Aldrich, 9 Diegem, Belgium), 80% bidest H20) and then incubated in 30% sucrose (Sigma-Aldrich, Diegem, Belgium) in phosphate-buffered saline (PBS) for 24 hours at 4°C. Next, the biopsies were frozen in Tissue-Tek® OCT™ Compound (Sakura, the Netherlands) on dry ice. Ten-micrometer sections were cut using a cryostat and dried on positively charged microscope slides for 2 hours at room temperature. The sections were then incubated with 5% donkey serum and 0.1% Triton X-100 in PBS for 2 hours, followed by sheep anti-human PGP9.5 (PH164, 1:10000, The Binding Site, Birmingham, UK) and rabbit anti-human VR1 (sc-28759, 1:1000, Santa Cruz Biotechnology) overnight at 4°C. Next, sections were incubated with secondary antibodies donkey anti-rabbit-Cy3 (1:1000, Chemicon) and donkey anti-sheep-FITC (1:500, Chemicon) for 2 hours at room temperature in the dark. The images were obtained using a laser-scanning confocal microscope (Leica, Wetzlar, Germany). PGP-positive and TRPV1 positively labeled nerve fibers were identified with a fluorescence microscope (BX61WI, Olympus, Hamburg) that was equipped with appropriate filter blocks and a black and white video camera (FView II, Olympus). Quantification was done in a random selection of biopsies with the cell^p software (Olympus Soft Imaging Solutions, Hamburg). All preparations were treated as follows: Three z-stacks were taken at 40x magnification in the mucosal and submucous layer for the PGP and TRPV1 staining. Identical z-stacks were taken using the Cy-5 filter block to determine autofluorescent structures. For the identification of specifically labeled fibers all z-stacks were processed with the “Extended Focal Imaging” option of the cell^p software. The area of PGP and TRPV1 positive fibers was determined in a field of view that did not contain any holes or damaged parts of the section by applying a threshold filter to the images. Areas with non-specific staining were excluded manually by comparison with the Cy-5 image, and TRPV1 positive fibers were only included for quantification calculation when they co-localized with PGP. Finally, areas were expressed as percentages of the entire field of view. The area of TRPV1 positive fibers was also expressed as percentage of PGP positive fibers. Data analysis and statistics 10 Study subjects were considered hypersensitive to rectal distension (VH) if discomfort or pain was reported during one of the three distensions steps. If this endpoint was reached during the second step (+9mmHg), the third step (+21 mm Hg) was not performed. For the statistical analysis, single point mutation was used to define values as 100 (maximum score) for the skipped distensions by last values carried forward principle. Urge (but not pain) scores during sham applications in VH-IBS patients were significantly higher compared with both ns-IBS patients and healthy volunteers suggesting a possible role of anticipation or increased awareness of the proctoscope. To avoid interference with interpretation of the data, a baseline correction of sensation scores during capsaicin applications was performed. Corrected scores were obtained by subtraction of scores during sham application from scores during real capsaicin application. During the course of the study, we noticed that some subjects suffered from persisting discomfort during 0.1% capsaicin applications continuing for several minutes after withdrawal of the application. Therefore we considered it unethical to continue the high (0.1%) capsaicin applications; as a consequence a small group of patients in the study (N=15) received only the 0.01% capsaicin application. State anxiety scores before and during the testing were normally distributed in each of the three groups and were analyzed using a mixed 2 x 3 two-way ANOVA (“time” before vs during testing, within-subject and “group” HV, NS-IBS, VH-IBS, between-subject), with Bonferroni corrected post-hoc t-tests. The baseline corrected urge and pain ratings were not normally distributed, therefore all analyses (oneway ANOVA to compare the three groups, ANCOVA to add the effect of anxiety to the group comparison) were done on ranks. Continuous data were compared using Student’s t-test and Mann– Whitney U-test (no difference between parametric or nonparametric testing), and categorical data using Chi-square tests. Differences were considered significant at the 5% level. Correlations between two parameters were performed using Spearman rank correlation. Statistical evaluations were performed using commercially available software (SPSS 18.0; SPSS Inc., Chicago,IL, USA) and SAS 9.3, SAS Institute, Cary, NC, USA). 11 Results: Study subjects: Forty-eight IBS patients fulfilling ROME III criteria and twenty-five healthy volunteers (HVs) were included. Age and gender did not differ between IBS patients (68% female, mean age 33±2, range 1860 years) and HVs (64% female, mean age 29±2, range 18-52 years). 26 patients with alternating IBS (IBS-M, 54%), 13 with diarrhea predominant IBS (IBS-D, 27%) and 8 with constipation predominant IBS (IBS-C, 17%) were included. One patient was classified as IBS-U (2%) (Table 1). Sensitivity to Rectal Distensions The maximal discomfort score was reached by 5 IBS patients during the 9 mmHg distension and by another 18 IBS patients during the 21 mmHg distension. These 23 IBS patients (48%) were categorized as hypersensitive to rectal distension (VH-IBS), the remaining 25 IBS patients (52%) were considered as normosensitive to rectal distension (ns-IBS). All HVs had a normal sensitivity for rectal distension. Mean VAS scores during each distension step for the different groups are presented in Table 1. Sensitivity to rectal mucosal capsaicin applications Healthy volunteers: Capsaicin (0.1-0.01%) application induced a dose-dependent increase in pain and urge intensity perceived by HVs (Fig. 2). In accordance, the number of controls perceiving any pain or urge increased dose-dependently. Perception of warmth and burning sensations showed a slight nonsignificant increase during application of 0.1% capsaicin. The perception of warmth and burning following rectal capsaicin application varied largely within subjects and were therefore considered non-reliable parameters and will not be further analyzed. IBS patients: 12 Also in IBS patients, capsaicin resulted in a dose-dependent increase in intensity of perceived pain and urge, and the number of patients with any pain or urge. Pain perception during 0.01% capsaicin application (one-way ANOVA on ranks F1,71 = 4.90, p=0.03) was increased in IBS patients compared to HVs; a trend towards such increase was found during 0.1% capsaicin application (one-way ANOVA on ranks F1,56 = 4.90, p=0.079). Urge perception was similar in IBS patients and HVs for both concentrations (data not shown). Hypersensitive and normosensitive IBS patients: A similar dose-dependent increase in pain and urge perception during capsaicin applications (0.010.1%) is present in the subgroups of VH-IBS and ns-IBS patients. However, the increase is only significant for the VH-IBS group. The percentage of subjects with any pain or urge was higher in both IBS patients subgroups compared to HVs. Pain perception triggered by 0.1% capsaicin application is significantly increased in VH-IBS patients compared to HVs (Fig. 2) (one-way ANOVA on ranks F2,55 = 5.14, p=0.009). Post-hoc t-tests with Tukey correction indicate a significant difference between VHIBS and HV (p=0.01 after correction) as well as ns-IBS (p=0.03 after correction), whereas there is no difference in pain perception between ns-IBS patients and HVs. A trend was found for the 0.01% capsaicin application condition (one-way ANOVA on ranks F2,70 = 2.74, p=0.071. Post-hoc t-tests with Tukey correction indicate a trend for the difference between VH-IBS and HV (p=0.06 after correction)). Urge perception is not different between HVs or IBS subgroups during any application. Rectal TRPV1 expression Rectal biopsies of 20 IBS patients and 8 HVs were evaluated for TRPV1 expression. The clinical characteristics of these patients were comparable to that of the whole IBS group (Table 2). Immunoreactive TRPV1 fibers were present throughout the mucosa, and much more abundant throughout the submucous plexus (Fig. 3). Quantification did not reveal any difference in median 13 number of mucosal TRPV1 fibers between the total IBS patients, VH-IBS (n=7), ns-IBS (n=13) and HVs. Similar, the number of TRPV1 fibers in the submucous plexus did not differ between IBS patients and HVs or hyper- and normosensitive subgroup of IBS patients (Fig. 4B). To correct for the number of nerve fibers present in the mucosa, we also performed immunohistochemical stainings for the neuronal marker PGP9.5. PGP9.5 expression was similar in IBS (n=20), HVs (n=8) and the VH-IBS (n=7) and ns-IBS (n=13) subgroups (Fig. 4A+B). In addition to immunohistochemistry, levels of mucosal TRPV1 and PGP9.5 mRNA expression in rectal biopsies were determined. In accordance with the protein expression data, there was no difference in TRPV1 or PGP9.5 mRNA expression between IBS patients and HVs (n=22), or the VH-IBS (n=18) and ns-IBS (n=24) subgroup of IBS patients (Fig. 4C). In subjects with an abnormal response to capsaicin (0.01% or 0.1%), TRPV1 and PGP9.5 mRNA expression levels were not different from those with a normal response (data not shown). Correlations between TRPV1 expression, pain response to capsaicin and clinical IBS symptoms To evaluate the role of TRPV1 in the symptom generation, the correlation between TRPV1 expression and the pain response or clinical IBS symptoms was calculated. Pain perception during 0.1% capsaicin applications was significantly associated with symptom scores of abdominal pain (r=0.4, p=0.024, Spearman correlation, N=32), loose stools (r=0.6, p<0.001, Spearman correlation, N=32) and increased bowel movements (r=0.5, p=0.004, Spearman correlation, N=32). For these symptoms there was no significant correlation with pain perception during 0.01% applications, (abdominal pain, r=0.3, p=0.084; loose stools, r=0.1, p=0.463, increased bowel movements, r=0.2, p=0.282, N=47) in IBS patients. Other clinical symptoms scored with the GSRS questionnaire did not correlate with the pain responses during 0.1% or 0.01% applications. Similarly, no significant correlations were found between TRPV1 expression levels (mRNA and protein) and pain perception during capsaicin application (0.1% and 0.01%). Finally, no significant correlations were found in subgroup analysis of 14 hyper- and normosensitive IBS patients between clinical symptoms, TRPV1 expression or capsaicininduced pain. Anxiety, depression and somatization scores Before the experimental procedure, subjects were asked to complete the STAI-state questionnaire for assessment of momentary (that is, in this case, anticipatory) anxiety. Further, immediately after the experimental procedure, study subjects were asked to report anxiety experienced during the procedure retrospectively on the same STAI-state questionnaire. First, we used a mixed 2 x 3 two-way ANOVA (“time” before vs during the experimental procedure, within-subject and “group” HV, NS-IBS, VH-IBS, between-subject), with Bonferroni corrected posthoc t-tests on state anxiety ratings before and during the procedure. This analysis revealed a significant “time”-by”group” interaction effect (F2,70=4.04, p=0.02), besides significant main effects of both “time” (F1,70=6.79, p=0.011) and “group” (F2,70=6.89, p=0.002). Post-hoc t-tests with Bonferroni correction indicated that there was a significant difference in anxiety ratings prior to the start of the experimental procedure between VH-IBS (40.1±2.0) and healthy controls (32.6±1.9) (p=0.044 after correction) and a trend between NS-IBS (39.3±1.9) and controls (p=0.085 after correction). Further, the increase in anxiety from before to during the experimental procedure was significant in VH-IBS only (40.1±2.0 vs 45.9±2.0, p=0.006 after correction) (Fig. 5). This indicates higher levels of anticipatory anxiety in IBS patients, and particularly in VH-IBS patients, compared to controls, and an increase in anxiety induced by the experimental procedure in VH-IBS only. Second, adding the change in anxiety (during – before the experimental procedure) as a continuous covariate to the model comparing the three groups in terms of pain response to the 0.1% capsaicin application demonstrated that both “group” (p=0.03) and anxiety (β=0.62, p=0.008) are independently associated with the pain response, jointly explaining 26% of the variance (ANCOVA on ranks, model F3,54=6.34, p<0.001, R²=0.26). 15 Somatization scores assessed by the PHQ-15 questionnaire were significantly higher in both IBS subgroups in comparison with HVs, whereas HADS depression scores did not differ between IBS subgroups and HVs (PHQ-15 somatization: VH-IBS 10±6.3, ns-IBS 9.6±5.0, HVs 5.1±2.8; p<0.05 and HADS depression: VH-IBS 2.6±3.7, ns-IBS 2.9±3.6, HVs 1.3±1.4, mean±SD). Discussion: In the present study, we evaluated the role of TRPV1 in hypersensitivity to rectal distension (referred to as “visceral hypersensitivity”) in IBS patients. We showed that patients with visceral hypersensitivity reported higher levels of anxiety during the procedure as well as increased subjective pain responses to rectal application of the TRPV1 agonist capsaicin compared to healthy controls and normosensitive IBS patients. Both anxiety during the procedure and visceral hypersensitivity were independently associated with the subjective pain response to capsaicin. Interestingly, an increased capsaicin-induced pain response was associated with the clinical symptoms of abdominal pain, loose stools and stool frequency. Our data indicate that anxiety or, more generally, central factors are involved in the increased pain response to rectal capsaicin in IBS patients. Moreover, as visceral hypersensitivity was associated with increased pain responses to capsaicin independently of anxiety, peripheral factors may also be involved with increased TRPV1 sensitivity as a speculative possibility, rather than upregulation of the receptor, which could not be demonstrated in our study. Although TRPV1 is classically described as a nociceptor activated by heat (above 42˚C), acidosis (pH < 6), and endovanilloids, abundant evidence has been reported favoring a role in visceral perception. Mice lacking TRPV1 have reduced perception of colorectal distention (24;31), whereas TRPV1 antagonists restore perception in animal models of visceral hypersensitivity (17;18;22;23;32). Moreover, mice that are hypersensitive to colorectal distension following water avoidance stress or 16 neonatal intracolonic infusion of acetic acid have increased expression of TRPV1 in the DRG neurons innervating the colon (18;32). To date, the evidence in humans supporting a role for TRPV1 in visceral perception is however limited to indirect evidence demonstrating increased TRPV1-expressing nerve fibers in rectal biopsies from patients with IBS and inflammatory bowel disease (25;33). These authors reported a threefold increase in the number of TRPV1-expressing nerve fibers, and a twofold increase in PGP9.5 in biopsies of IBS patients. In the present study, we reasoned that TRPV1 expression would be particularly increased in IBS patients with proven hypersensitivity to rectal distension. Surprisingly, however, no differences in mRNA levels of TRPV1 could be demonstrated in rectal biopsies of patients with IBS compared to controls. This observation was confirmed by immunohistochemical stainings showing no difference in TRPV1- and PGP9.5-expressing nerve fibers. These data are in strong contrast to the previous findings reported by Akbar et al (25). Possible explanations for this discrepancy may be differences in patient selection. In the Akbar study, increased numbers of mast cells and CD3+ T cells were observed, suggesting the presence of microscopic inflammation. The latter, via release of NGF or other growth factors by mast cells, may explain a possible contribution of nerve sprouting (increased PGP9.5) and increased TRPV1 expression. As previously reported, we fail to confirm microscopic inflammation in our IBS population probably explaining the absence in TRPV1 upregulation (34). Alternatively, differences in TRPV1 antibody used for immunohistochemical staining could be involved. It should indeed be emphasized that the specificity of the TRPV1 antibodies currently available is questionable. We have tested 16 different TRPV1 antibodies (data not shown), including the one used by Akbar et al., and found nonspecific staining in all except with the antibody used here. Hence, immunohistochemical TRPV1 data should be interpreted with caution. Another limitation is that TRPV1 immunohistochemical staining was only performed in a subset of biopsies. The fact that we also found no differences in TRPV1 mRNA levels seems to support our finding that TRPV1 is not upregulated in rectal mucosal biopsies of IBS. However, although there is evidence that mRNA is present and transported to the peripheral nerve endings, we cannot exclude that different processes take place in the cell bodies of the sensory 17 nerves ((35;36). To what extent TRPV1 may be upregulated in deeper layers of the intestinal wall remains unclear. The latter may be relevant given the fact that perception evoked by distension, as assessed by barostat, is most likely triggered by activation of receptors located in the muscular or serosal layers (15). Alternatively, other receptors such as TRPV4, acid sensing ion channels (ASICs) and transient receptor potential ankyrin 1 (TRPA1) may be involved in development of hypersensitivity to rectal distension (37-39). Although we were unable to detect differences in TRPV1 expression, patients with visceral hypersensitivity experienced more pain in response to rectal capsaicin application compared to normosensitive IBS patients and healthy subjects and this was shown to be independent of anxiety levels, although the latter were also significantly associated with an increased pain response. Mucosal application of capsaicin has been previously proposed as an acceptable model to study visceral pain (26). Low dose capsaicin applications to healthy ileal and colonic mucosa evoked nonpainful diffuse sensations (warm or non-specific sensations), whereas application of higher doses (median 0.5ml of 50 μg/ml solution) capsaicin evoked a painful sensation after approximately 45 seconds (26;27). Similarly, in our study, healthy controls only reported pain in response to application of 0.1% capsaicin, but not of 0.01%, to the rectal mucosa. In contrast, however, IBS patients reported pain at the lower concentration further increasing in intensity with 0.1% capsaicin. Of note, no desensitization was observed as the different applications were applied at different sites in the rectum at least one cm apart. Most interestingly, however, patients with proven hypersensitivity to rectal distension had significantly higher pain scores compared to normosensitive IBS patients and healthy controls. Moreover, we observed a correlation between the pain scores evoked by capsaicin and the pain scores assessed using the GSRS questionnaire. In addition to pain, rectal capsaicin application also induced a concentration-dependent increase in the sensation of urge to defecate. Remarkably, IBS patients reported increased perception of urge to 18 defecate in response to capsaicin compared to healthy controls, but this difference was already apparent during sham application. After subtraction of the urge scores induced by sham application, however, no differences were observed anymore, suggesting that anticipation may be involved in the increased rating of urge by IBS patients. The finding that the anxiety scores before the experimental procedure were higher in hypersensitive IBS patients supports the idea that central factors or anticipation may have contributed to the increased perception during sham application. Indeed, both anxiety and stimulus expectation have been associated with differences in central processing of peripheral stimuli (13;40;41). Of interest, anxiety levels were higher before the procedure in IBS patients, indicating higher levels of anticipatory anxiety. Moreover, anxiety levels increased further significantly during the procedure in VH-IBS patients, indicating a higher increase in anxiety induced by the procedure or alternatively by the more severe evoked pain response in this subgroup of patients. Based on our data, it is impossible to demonstrate which of these two interpretations apply, as anxiety levels were measured retrospectively after completion of the whole procedure. However, anxiety levels before the start of the procedure were similar between VH- and NS-IBS groups, rendering it somewhat more unlikely that anticipatory anxiety is playing an important role. Taken together, these data indicate that central factors, especially anxiety, are involved in the increased pain response to capsaicin observed in hypersensitive IBS patients, independently of visceral hypersensitivity. In addition to central factors, we speculate that peripheral factors may also contribute. Indeed, , when anxiety was added as a continuous covariate to the model comparing the pain response to capsaicin between groups, both visceral sensitivity and anxiety appeared independently associated with pain evoked by capsaicin, jointly explaining 26% of the variance in pain response. These data suggest that although anxiety is indeed an important modulator of visceral pain perception, visceral hypersensitivity is independently associated with an increased pain response triggered by selective TRPV1 activation in the rectal mucosa. Although we failed to demonstrate upregulation of TRPV1 in the rectal biopsies, this finding does not necessarily excludes a role of this nociceptor in visceral 19 hypersensitivity. For example, in a rat model of visceral hypersensitivity, treatment with the TRPV1 antagonist SB-705498 reversed increased pain perception evoked by water avoidance stress, yet no upregulation of TRPV1 could be demonstrated (22). These data suggest that sensitization of TRPV1 by inflammatory mediators or mast cell mediators rather than upregulation may be underlie visceral hypersensitivity. The potential to sensitize TRPV1 has been shown for a large group of mediators, including prostaglandins, bradykinin, 5-HT, nerve growth factor (NGF), lipoxygenase products (20;21) and an inflammatory soup containing the mast cell mediators 5-HT and histamine together with bradykinin (24). Especially as mast cells have been implicated in the pathogenesis of IBS, sensitization of TRPV1 by mast cell mediators could be involved in the increased pain response to rectal capsaicin application. To what extent this may (partly) explain the beneficial effect of the mast cell stabilizer ketotifen in IBS patients (5), remains to be proven. Our study has some limitations. First, it should be emphasized that this is an exploratory study rather than hypothesis-testing study. Hence, our data should be interpreted with care and should be rather regarded as hypothesis generating. In addition, for some of the participants, the study protocol was too demanding, such that no biopsies could be collected from 3 HV and 8 IBS patients. Finally, immunohistochemical stainings were only performed in a subpopulation of patients. In conclusion, although no upregulation of TRPV1 could be demonstrated, IBS patients with VH to rectal distension reveal increased pain perception and an increased anxiety response to rectal application of capsaicin. As both visceral hypersensitivity and anxiety are independently associated with increased pain perception to rectal capsaicin application, our data suggest that both peripheral and central factors are involved, with increased receptor sensitivity as a speculative possibility. 20 Reference List (1) Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology 2002 December;123(6):2108-31. (2) Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010 March;7(3):163-73. (3) Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De GR, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil 2011 October;17(4):349-59. (4) Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2005 July 15;22(2):157-64. (5) Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010 September;59(9):1213-21. (6) Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008 July;6(7):772-81. (7) Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 1994 December;107(6):1686-96. (8) Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 1995 July;109(1):40-52. (9) Sabate JM, Veyrac M, Mion F, Siproudhis L, Ducrotte P, Zerbib F, Grimaud JC, Dapoigny M, Dyard F, Coffin B. Relationship between rectal sensitivity, symptoms intensity and quality of life in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2008 August 15;28(4):484-90. (10) Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 2007 October;133(4):1113-23. (11) van der Veek PP, Van Rood YR, Masclee AA. Symptom severity but not psychopathology predicts visceral hypersensitivity in irritable bowel syndrome. Clin Gastroenterol Hepatol 2008 March;6(3):321-8. (12) Van OL, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Demyttenaere K, Fischler B, Tack J. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med 2007 June;69(5):455-63. (13) Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 2010 April;59(4):489-95. (14) Anand P, Aziz Q, Willert R, van OL. Peripheral and central mechanisms of visceral sensitization in man. Neurogastroenterol Motil 2007 January;19(1 Suppl):29-46. (15) Feng B, La JH, Schwartz ES, Gebhart GF. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012 May 15;302(10):G1085-G1098. (16) Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010 November;16(11):1248-57. 21 (17) De Schepper HU, De Man JG, Ruyssers NE, Deiteren A, Van NL, Timmermans JP, Martinet W, Herman AG, Pelckmans PA, De Winter BY. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am J Physiol Gastrointest Liver Physiol 2008 January;294(1):G245-G253. (18) Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 2007 February;132(2):615-27. (19) Hughes PA, Brierley SM, Martin CM, Liebregts T, Persson J, Adam B, Holtmann G, Blackshaw LA. TRPV1-expressing sensory fibres and IBS: links with immune function. Gut 2009 March;58(3):465-6. (20) Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 2007 May;6(5):357-72. (21) Holzer P. TRPV1: a new target for treatment of visceral pain in IBS? Gut 2008 July;57(7):8824. (22) van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der Coelen D, Bulmer DC, Peeters PJ, Aerssens J, de HR, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil 2009 June 11. (23) Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007 September 21;148(4):1021-32. (24) Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 2005 November 23;25(47):10981-9. (25) Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008 July;57(7):923-9. (26) Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, ArendtNielsen L. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain 2003 July;104(1-2):333-41. (27) Arendt-Nielsen L, Schipper KP, Dimcevski G, Sumikura H, Krarup AL, Giamberardino MA, Drewes AM. Viscero-somatic reflexes in referred pain areas evoked by capsaicin stimulation of the human gut. Eur J Pain 2008 July;12(5):544-51. (28) Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006 April;130(5):1480-91. (29) Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988 February;33(2):129-34. (30) Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001 May 1;29(9):e45. (31) Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol 2004 November 1;560(Pt 3):86781. (32) Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 2009 February;58(2):202-10. (33) Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010 June;59(6):767-74. (34) Braak B, Klooker TK, Wouters MM, Welting O, van der Loos CM, Stanisor OI, van DS, van den Wijngaard RM, Boeckxstaens GE. Mucosal immune cell numbers and visceral sensitivity in 22 (35) (36) (37) (38) (39) (40) (41) patients with irritable bowel syndrome: is there any relationship? Am J Gastroenterol 2012 May;107(5):715-26. Meer EJ, Wang DO, Kim S, Barr I, Guo F, Martin KC. Identification of a cis-acting element that localizes mRNA to synapses. Proc Natl Acad Sci U S A 2012 March 20;109(12):4639-44. Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of processlocalized mRNAs from cultured rodent hippocampal neurons. J Neurosci 2006 December 20;26(51):13390-9. Cenac N, Altier C, Motta JP, D'Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 2010 April;59(4):481-8. Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 2005 October;54(10):1408-15. Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 2011 July 15;589(Pt 14):3575-93. Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci 2008 January 9;28(2):349-59. Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 2006 December 15;126(1-3):7990. 23 Figure Legends Figure 1. Schematic presentation of study protocol Figure 2. Urge and pain perception during rectal capsaicin applications Urge perception increased concentration dependently in VH-IBS, ns-IBS and HVs, but did not differ between these groups (A). Pain perception increased dose dependently in VH-IBS, ns-IBS and HVs. During 0.1% applications pain perception in VH-IBS was significantly increased compared to HVs and ns-IBS. Data were analyzed by one-way ANOVA on ranks (F2,55 = 5.14, p=0.009) and group comparisons by post-hoc t-tests with Tukey correction. There was a similar, but non-significant trend during the 0.01% applications (F2,70 = 2.74, p=0.071) (B). *p<0.05 VH-IBS vs HVs and ns-IBS Figure 3. Immunohistochemical stainings of TRPV1 and PGP9.5 White arrows indicate sites of TRPV1 and PGP9.5 co-localization on afferent neurons. This figure shows representative pictures of healthy volunteers (left) and IBS patients (right). scale bar = 100 μm Figure 4. Rectal TRPV1 and PGP9.5 expression TRPV1, PGP9.5 and ratio TRPV1/PGP 9.5 is presented for mucosal protein expression (4A), submucous protein expression (4B) and mucosal mRNA expression (4C), each dot represents an individual, and lines represent the median. Figure 5. State anxiety scores before and during the experimental procedure Before: State anxiety scores in VH-IBS patients were significantly higher compared to HVs, but were not different in VH-IBS and ns-IBS (**F2,70=6.89, p=0.002, after post-hoc t-test with Bonferroni correction: p=0.044 for comparison of VH-IBS and HVs). During: Anxiety scores significantly increased in VH-IBS during the sensitivity test compared to before(*F1,70=6.79, p=0.011, and p=0.006 after correction in post-hoc t-test) while this effect was not seen in ns-IBS or HVs. Each dot dots represents an individual, lines represent mean with standard error of the mean. STAI-state = State-Trait Anxiety Inventory, state version. 24 CONFLICTS OF INTEREST Guarantor of the article: Guy E. Boeckxstaens, MD, PhD Specific author contributions: Sander JM van Wanrooij: study concept and design, acquisition of data, analysis and interpretation of data, writing the manuscript Mira M Wouters: data acquisition, analysis and interpretation of data, critical revision of the manuscript for important intellectual content Lukas Van Oudenhove: statistical analysis, interpretation of data, critical revision of the manuscript for important intellectual content Winde Vanbrabant: recruitment of study subjects, data acquisition Stéphanie Mondelaers: data acquisition Patrick Kollmann: data acquisition Florian Kreutz: data acquisition Michael Schemann: supervision TRPV1 immunohistochemistry analysis, critical revision of the manuscript for important intellectual content Guy Boeckxstaens: study supervision, obtaining funding, critical revision of the manuscript for important intellectual content Financial support: Guy Boeckxstaens received research funding by a grant from the Flemish government (Odysseus Program, FWO). Michael Schemann received a funding by the Deutsche Forschungsgemeinschaft (DFG Sche/267/7-2). Mira Wouters received a postdoctoral fellowship (FWO) from the Flemish government. This work was supported by research grant G.0699.10N from the FWO. Potential competing interests: None 25