Hess' Law Worksheet: Thermochemistry Practice Problems
advertisement
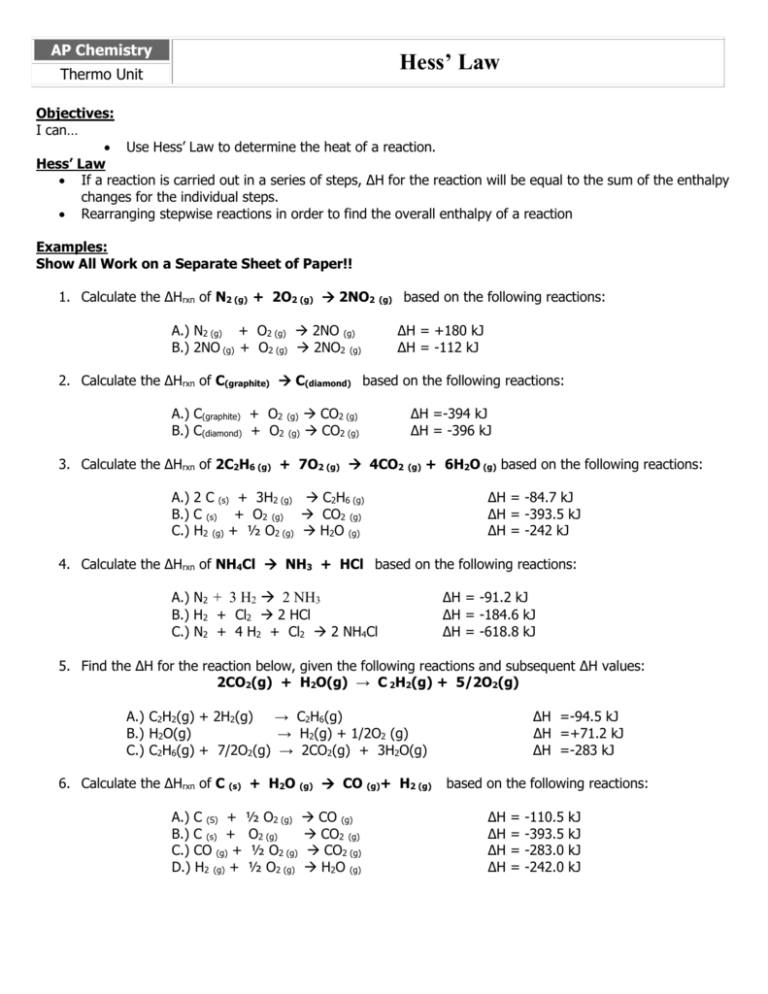
AP Chemistry Hess’ Law Thermo Unit Objectives: I can… Use Hess’ Law to determine the heat of a reaction. Hess’ Law If a reaction is carried out in a series of steps, ΔH for the reaction will be equal to the sum of the enthalpy changes for the individual steps. Rearranging stepwise reactions in order to find the overall enthalpy of a reaction Examples: Show All Work on a Separate Sheet of Paper!! 1. Calculate the ΔHrxn of N2 (g) + 2O2 (g) 2NO2 (g) based on the following reactions: A.) N2 (g) + O2 (g) 2NO (g) B.) 2NO (g) + O2 (g) 2NO2 (g) ΔH = +180 kJ ΔH = -112 kJ 2. Calculate the ΔHrxn of C(graphite) C(diamond) based on the following reactions: A.) C(graphite) + O2 (g) CO2 (g) B.) C(diamond) + O2 (g) CO2 (g) ΔH =-394 kJ ΔH = -396 kJ 3. Calculate the ΔHrxn of 2C2H6 (g) + 7O2 (g) 4CO2 (g) + 6H2O (g) based on the following reactions: A.) 2 C (s) + 3H2 (g) C2H6 (g) B.) C (s) + O2 (g) CO2 (g) C.) H2 (g) + ½ O2 (g) H2O (g) ΔH = -84.7 kJ ΔH = -393.5 kJ ΔH = -242 kJ 4. Calculate the ΔHrxn of NH4Cl NH3 + HCl based on the following reactions: A.) N2 + 3 H2 2 NH3 B.) H2 + Cl2 2 HCl C.) N2 + 4 H2 + Cl2 2 NH4Cl ΔH = -91.2 kJ ΔH = -184.6 kJ ΔH = -618.8 kJ 5. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: 2CO2(g) + H2O(g) → C 2H2(g) + 5/2O2(g) A.) C2H2(g) + 2H2(g) → C2H6(g) B.) H2O(g) → H2(g) + 1/2O2 (g) C.) C2H6(g) + 7/2O2(g) → 2CO2(g) + 3H2O(g) 6. Calculate the ΔHrxn of C (s) + H2O (g) CO (g)+ H2 (g) A.) C (S) + ½ O2 (g) CO (g) B.) C (s) + O2 (g) CO2 (g) C.) CO (g) + ½ O2 (g) CO2 (g) D.) H2 (g) + ½ O2 (g) H2O (g) ΔH =-94.5 kJ ΔH =+71.2 kJ ΔH =-283 kJ based on the following reactions: ΔH ΔH ΔH ΔH = = = = -110.5 -393.5 -283.0 -242.0 kJ kJ kJ kJ 7. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: NO (g) + O (g) NO2 (g) A.) B.) C.) 8. NO (g) 2 O3 (g) 3 O2 (g) O2 (g) 2 O (g) + O3 (g) NO2 (g) + O2 ΔH = - 427 kJ ΔH = + 495 kJ ΔH = - 199 kJ (g) Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: CO2 (g) → C (s) + O2 (g) A.) H2O (l) → H2 (g) + ½ O2 (g) B.) C2H6 (g) → 2 C (s) + 3 H 2 (g) C.) 2 CO2 (g) + 3 H2O (l) → C 2H6 (g) + 7/2 O2(g) ΔH = + 286 kJ ΔH = - 190.6 kJ ΔH = + 3511.1 kJ 9. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: ½ H2 (g) + ½ Cl2 (g) → HCl (g) A.) B.) C.) CH2Cl2 (l) COCl2 (g) + H2O (l) → CH2Cl2 (l) + O2 (g) 2 HCl (g) + ½ O2 (g) → H2O (l) + Cl2 (g) + H2 (g) + 3/2 O2 (g) → COCl2 (g) + 2 H2O ΔH = + 47.5 kJ ΔH = +105 kJ ΔH = -402.5 kJ (l) 10. Calculate the ΔHrxn of Fe3O4 (s) + CO (g) 3 FeO (s) + CO2 (g) based on the following reactions: A.) 2 Fe (s) + 3 CO2 (g) Fe2O3 (s) + 3 CO (g) B.) 2 Fe3O4 (s) + CO2 (g) 3 Fe2O3 (s) + CO (g) ΔH = - 23 kJ ΔH = - 39 kJ C.) FeO (s) + CO (g) Fe (s) + CO2 (g) ΔH = + 18 kJ 11. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: HCl (g) + NaNO2 (s) → HNO2 (l) + NaCl (s) A.) B.) C.) D.) NO (g) 2 NaCl (s) + H2O (l) → 2 HCl (g) + Na2O (s) + NO2 (g) + Na2O (s) → 2 NaNO2 (s) NO (g) + NO2 (g) → N2O (g) + O2 (g) 2 HNO2 (l) → N2O (g) + O2 (g) + H2O ΔH = + 507 kJ ΔH = - 427 kJ ΔH = - 43 kJ ΔH = + 34 kJ (l) 12. Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: P4O10 (s) + 6 PCl5 (g) 10 Cl3PO (g) A.) B.) C.) D.) P4 (s) + 6 Cl2 (g) 4 PCl3 (g) P4 (s) + 5 O2 (g) P4O10 (s) PCl3 (g) + Cl2 (g) PCl5 (g) PCl3 (g) + ½ O2 (g) Cl3PO (g) ΔH ΔH ΔH ΔH = = = = - 1225.6 kJ 2967.3 kJ 84.2 kJ 285.7 kJ