Supplementary Information
advertisement

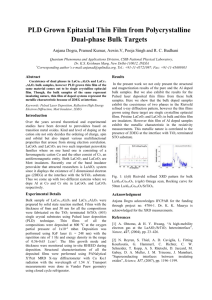
Supporting Information for Ultrafast Atomic Layer-by-Layer Oxygen Vacancy-Exchange Diffusion in DoublePerovskite LnBaCo2O5.5- Thin Films ShangyongBao, Chunrui Ma, Garry Chen, Xing Xu, Erik Enriquez, and Chonglin Chen* Department of Physics and Astronomy, University of Texas at San Antonio, TX 78249, USA Yamei Zhang Department of Physics, Jiangsu University of Science and Technology, Zhenjiang, Jiangsu 212003, China Jerry L. Bettis, Jr. and Myung-Hwan Whangbo Department of Chemistry, North Carolina State University, Raleigh, NC 27695-8204, USA Chuang Dong and Qingyu Zhang Key Laboratory of Materials Modification by Laser, Ion and Electron Beams, Ministry of Education, Dalian University of Technology, Dalian 116024, China. 1. Experimental Under the laser energy density of about 1.5 - 2.0 J /cm2 at a repetition rate of 5 Hz, good single crystalline LnBCO epitaxial films are grown on (001) LaAlO3 substrates under the optimized growth condition of an oxygen pressure of 250 mTorr at 850 C, similar to that for the growth of other LaBCO epitaxial thin films, as shown in Fig. S1. Details of the optimized film growth were presented in the previous studies.i,ii,iiiThe crystallinity and epitaxial nature were checked by XRD and confirmed by TEM microscopy. The XRD measurements indicate that the all epitaxial films are c-axis oriented, namely, the ab-plane of the ErBCO lattice is parallel to the substrate surface. The redox reactions on the ErBCO films were conducted by using a Lake Shore 370 AC Precise Resistance Bridge System in the range of 200 - 700oCunder a switching flow of O2 and H2 at 1 atm. Platinum leads were glued on the film surface with high temperature silver paste, which was air-dried at room temperature and annealed at 800 o C before measurements. Fig. S1 (a) x-ray diffraction, (b) cross sectional TEM image and diffraction pattern, iv (c) high resolution TEM image and EDX showing the excellent epitaxial nature of single crystalline LaBCO thin films on LAO.v (c) (a) (b) 2. R vs. t and dR/dt vs. t plots obtained for the A-site disordered LaSrCo2O5.5+ The resistance of single crystalline LaSCO thin films grown on (001) LaAlO3 was examined at various temperatures under the switching flow of the reducing (H2) and oxidizing (O2) gases. As shown in Fig. S2 for the results obtained at 300 C, oscillation peaks are not found in the dR/dt vs. t plot. The inset of Fig. S2 shows a zoomed-in view of the resistance change during t = 9810 9834 s. Figure S2. 3. Diffusion of carriers through thin layers of thickness L The carrier density c(x, t) at the distance x from the center of the layer (so that the distance between the center to the surface is L/2) at the time t is related to the diffusion coefficient D of the redox gas as followsvi c(x, t) c1 4(1)i x Dt 1 cos[(2i 1) ]exp[2 (2i 1) 2 2 c2 c1 L L i 0 (2i 1) (1) If the conductivity is dominated by one type of charge carrier, the mean conductance (x, t) is written as 1 8 1 t 1 2 exp[(2i 1)2 ] , 2 2 1 i 0 (2i 1) (2) where 1 and 2 are the conductance at the initial and final states, respectively, and is the relaxation time L2 2 . D (3) For small times (t <<), Eq. (2) is simplified as 1 t 43/2 . 2 1 (4) where 1 and 2 are the conductance at the initial and final states, respectively, and this expression is valid when t is small compared to the relaxation time conductance change during a short time interval t is given by L2 . Then, the 2 D C t , t where C 23/2 (2 1 ) 1/ . Using this expression we analyze the resistance oscillations observed during the oxidation cycle at various temperatures ranging from 240 to 400 oC to deduce the associated relaxation times , and subsequently the diffusion coefficients D by taking L/2 as the c-axis lattice constant. Table 1 summarizes our results obtained at various temperatures ranging from 240 to 400 oC. 4. Comparison of the diffusion rates The oxygen diffusion in ErBCO is faster than that in YSZ, Gd:CeO2 and other similar perovskite oxides by several orders of magnitude,vii,viii,ix,x while the hydrogen diffusion in ErBCO is comparable in rate to the silver diffusion in -Ag2+S and -Ag2Te.xi References i Yuan, Z., Liu, J., Chen, C. L., Wang, C. H., Luo, X. G., Chen, X. H., Kim, G. T., Huang, D. X., Wang, S. S., Jacobson, A. J. & Donner, W. Epitaxial behavior and transport properties of PrBaCo2O5 thin films on (001) SrTiO3. Appl. Phys. Lett. 90, 212111 (2007). ii Liu, M., Ma, C. R., Liu, J., Collins, G., Zhang, Y. M., Chen, C. L., He, J., Jiang, J. C., Meletis, E.I., Lin, Y., Sun, L., Jacobson, A. J. & Zhang, Q. Y. Giant Magnetoresistance and Anomalous Magnetic Properties of Highly Epitaxial Ferromagnetic LaBaCo2O5.5+δ Thin Films on (001) MgO. Appl. Mater. Interface 4, 5524 (2012). iii Ma, C. R., Liu, M., Collins, G., Wang, H. B., Bao, S. Y., Xu, X., Chen, C. L., Enriquez, E., Lin, Y. & Whangbo, M.-H. Magnetic and Electrical Transport Properties of LaBaCo2O5.5+δ Thin Films on Vicinal (001) SrTiO3 Surfaces. Appl. Mater. Interface 5, 451 (2013). Liu, J., Liu, M., Collins, G., Chen, C. L., Jiang, X. N., Gong, W. Q., Jacobson, A. J., He, J., iv Jiang, J. C., and Meletis, E. I., “Epitaxial Nature and Transport Properties in Double Perovskite LaBaCo2O5.5+ Thin Films”, Chem. Mat., 22, 799, 2010 v Ruiz-Zepeda, F., Ma, C. R., Bahena Uribe, D., Cantu-Valle, J., Wang, H. B., Xu, X., Yacaman, M. J., Chen, C. L., Lorenz, B., Jacobson, A. J., Chu, P. C. W., and A. Ponce, “Nanodomain Induced Anomalous Magnetic and Electronic Transport Properties of LaBaCo2O5.5+ Highly Epitxial Thin Films”, J Appl. Phys. 115, 024301, 2014 vi Maier, J. Physical Chemistry of Ionic Materials, Wiley, Chichester, p. 313 (2004). viiMinervini, L., Zacate, M. O. & Grimes, R. W. Defect cluster formation in M2O3-doped CeO2. Solid State Ionics 116, 339 (1999). viii Bridges, C. A., Fernandez-Alonso, F., Goff, J. P. & Rosseinsky, M. J. Observation of Hydride Mobility in the Transition-Metal Oxide Hydride LaSrCoO3H0.7. Adv. Mat. 18, 3304 (2006). ixTarancón, A., Skinner, S. J., Chater, R. J., Hernández-Ramírez, F. & Kilner, J. A. Layered perovskites as promising cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 17, 3175 (2007). x Maier, J. et al. Mass transport in the presence of internal defect reactions—Concept of conservative ensembles: I, chemical diffusion in pure Compounds. J. Am. Ceram. Soc. 76, 1212 (1993). xi J. Maier, Physical Chemistry of Ionic Materials, Wiley, Chichester, 2004, p. 318.