Special Populations: Children in Research
advertisement

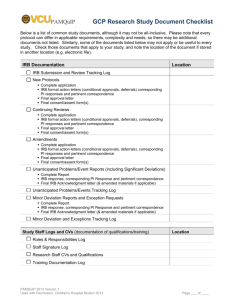
Last Revised: 10.2015 Prior Version: 7.2007 Special Populations in Research Children Introduction Research, including chart reviews, that involves children or the use of Protected Health Information (PHI) of children must meet the criteria for approval set forth in 45 CFR 46, Subparts A and D, unless the research has been determined to be exempt from IRB review. Please note that there are restrictions for approving exempt research involving children. When children are involved in research, federal regulations require the permission of the parent(s) or guardian before a child can be enrolled in research. While children may be legally incapable of giving informed consent, they nevertheless may possess the ability to assent, or refuse participation in a research study. Assent is a child's affirmative agreement to participate in research after an explanation of the study in language the child can understand. Failure to object to participation cannot be construed as assent. The following guidelines have been established by the IRB to assist investigators who conduct research involving children. Federal regulations require that assent must be sought from children unless the requirement is waived by the IRB or is not required under applicable regulations. Assent must be taken seriously by all investigators who include children as subjects of research. It is the IRB Chair’s responsibility to have the convened IRB or the IRB Manager to have the expedited reviewer(s) determine and document whether the following criteria for approval of research are met when research involves children. Category 1 (45 CFR 46.404): No greater than minimal risk to children is presented. Where parental permission is to be obtained, the IRB must declare whether one or both parents must give permission for the child’s research participation. Category 2 (45 CFR 46.405): More than minimal risk to children is presented by an intervention or procedure that holds out the prospect of direct benefit for the individual subject, or by a monitoring procedure that is likely to contribute to the subject’s well-being. The risk is justified by the anticipated benefit to the subjects. The relation of the anticipated benefit to the risk is at least as favorable to the subjects as that presented by available alternative approaches. Where parental permission is to be obtained, the IRB must declare whether one or both parents must give permission for the child’s research participation. Category 3 (45 CFR 46.406): Page 1 of 4 CWRU SBER IRB Policies and Procedures Last Revised: 10.2015 Prior Version: 7.2007 More than minimal risk to children is presented by an intervention or procedure that does not hold out the prospect of direct benefit for the individual subject, or by a monitoring procedure which is not likely to contribute to the well-being of the subject. The risk represents a minor increase over minimal risk. The intervention or procedure presents experiences to subjects that are reasonably commensurate with those inherent in their actual or expected medical, dental, psychological, social, or educational situations. The intervention or procedure is likely to yield generalizable knowledge about the subjects’ disorder or condition which is of vital importance for the understanding or amelioration of the subjects’ disorder or condition. Where parental permission is to be obtained, both parents must give permission unless one parent is deceased, unknown, incompetent, not reasonably available, or does not have legal responsibility for the care and custody of the child. Category 4 (45 CFR 46.407): Research not otherwise approvable and presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children. Requires approval by the Secretary of Health and Human Services, as applicable. The federal agency, after consultation with a panel of experts in pertinent disciplines (for example: science, medicine, education, ethics, law) and following opportunity for public review and comment, determined either: The research fell into categories 1 through 3; or The research presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children and the research will be conducted in accordance with sound ethical principles. Where parental permission is to be obtained, both parents must give permission unless one parent is deceased, unknown, incompetent, not reasonably available, or does not have legal responsibility for the care and custody of the child. It is the IRB Chair’s responsibility to have the convened IRB determine and document whether the following criteria for approval of research are met when research in Category 3(45 CFR 46.406) or 4 (45 CFR 46.407) involves wards of the state or any other agency. The research is: Related to their status as wards; or Conducted in schools, camps, hospitals, institutions, or similar settings in which the majority of children involves as subjects are not wards. Page 2 of 4 CWRU SBER IRB Policies and Procedures Last Revised: 10.2015 Prior Version: 7.2007 The IRB requires appointment of an advocate for each child who is a ward, in addition to any other individual acting on behalf of the child as guardian or in loco parentis. The advocate is an individual who has the background and experience to act in, and agrees to act in, the best interests of the child for the duration of the child’s participation in the research The advocate is not associated in any way (except in the role as advocate or member of the IRB) with the research, the investigators, or the guardian. Definitions Adult is a person who has attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted. Who is an adult may vary depending on the specific treatments or procedures involved in the research and on the jurisdiction in which the research will be conducted. Assent means a child’s affirmative agreement to participate in research. Failure of a child to object to participation cannot be construed as assent. Assent is a process involving communication with the child. A signature on an assent document is not, by itself, assent. Child is a person, who has not attained the legal age for consent to treatments or procedures involved in research, under the applicable law of the jurisdiction in which the research will be conducted. In most American states, a child is someone who is age 17 years old or younger. Other countries may define a child differently. For example, in Taiwan, a child is someone who is 19 years old or younger. Emancipated minor is a person under the legal age of majority who, usually via a court order, has the legal rights of an adult. Situations that qualify a person as an emancipated minor vary from state to state. In Ohio, a person under the legal age of majority becomes an emancipated minor by order of the court. Grounds for emancipation include marriage or service in the armed forces. Documentation of emancipation by court order is required before this doctrine can apply in the research context, in some situations copies of a court order may be required. Please note if a minor is a parent and has a child of her/his own that does NOT mean that she/he is emancipated. In this case, investigators would have to collect assent from the minor parent and parental consent from that minor’s parent/guardian. Guardian is an individual, who is legally authorized under applicable state or local law, to consent on behalf of a child to general medical care. Informed Consent is an individual’s voluntary agreement, based upon adequate knowledge and understanding of the relevant information, to participate in research either for themselves or for a child for whom they are the parent, guardian, or legally authorized representative. Page 3 of 4 CWRU SBER IRB Policies and Procedures Last Revised: 10.2015 Prior Version: 7.2007 Parent generally means a child’s biological or adoptive parent. Foster parents are not authorized to give research consent. Permission or Parental Permission means the agreement of the parent(s) or legally authorized guardian to the participation of a child in research. This term is often used to emphasize that the parent is not the subject of the research. In this context permission has the same meaning as consent. Policy It is a requirement that the investigator propose an assent plan as part of a research protocol that includes children as subjects. If the investigator believes that assent is not appropriate for the child population being studied, appropriate justification must be provided in the protocol. Requests for waivers of assent need to be specifically requested and subsequently approved by the IRB. The investigator must also describe the additional safeguards in place to protect the rights and welfare of the children. Please refer to “Assent from Children” References and/or Regulatory Citations: 45 CFR Subpart B 45 CFR 46.404 45 CFR 46.405 Most of the federal regulations relating to research in children are in 45 CFR 46.401-409, 45 CFR 46, Subpart C: Additional Protections Pertaining to Biomedical and Behavioral Research Involving Prisoners as Subjects OHRP Guidance Document: “OHRP Guidance on Involvement of Prisoners in Research,” May 23, 2003 OHRP Guidance: Children: Research with Children FAQ OHRP Guidance: Information on Special Protections for Children as Research Subjects Office for Human Research Protections (OHRP), Special Protections for Children as Research Subjects, Children Involved as Subjects in Research: Guidance on the HHS, 45 CFR 46.407 ("407") Review Process, May 26, 2005 Page 4 of 4 CWRU SBER IRB Policies and Procedures