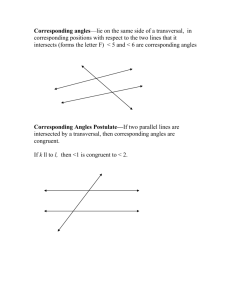
Supplementary Material
advertisement

Supplementary Material Local orientational entropy S in terms of density We show that Eq 3 for the local orientational entropy, S (r ) k B g sw ( | r ) ln g sw ( | r )d 8 2 can be written in terms of the orientational probability density, r (w | r) , instead of the orientational distribution, g(w | r). Since the relationship between g(w | r) and the orientational density r (w | r) is: gsw (w | r) º r(w | r) / rwo , where 1/ (8p 2 ) = rwo is the orientational density of a uniform orientational distribution, we can rewrite S in term of r (w | r) as: S (r ) k B ( | r ) ln 8 2 ( | r ) d k B ( | r ) ln ( | r ) d k B ( | r ) ln 8 2 d k B ( | r ) ln ( | r ) d k B ln 8 2 ( | r )d However, r (w | r) is normalized, so its integral ( ò r(w | r)dw ) equals unity. Hence, S is simply S (r ) k B ( | r ) ln ( | r )d k B ln 8 2 where the second term on the right signifies the orientational entropy of the uniform orientational distribution of a water molecule in the bulk: -kB ò r wo ln( r wo )dw = kB kB ln(8p 2 ) 2 ln(8 p ) d w = dw =kB ln(8p 2 ) . 2 ò 2 ò 8p 8p Hence, S (r ) k B ( | r ) ln ( | r) d k B o lno d . Definition of water Euler angles Figure S1. Definition of water Euler angles. The lab coordinate frame is shown in black, and the water frame in red. The water oxygen is at the origin. We use the x-convention77 to define the Euler angles of a water molecule’s internal coordinate frame relative to the grid’s coordinate frame (see Figure). A water molecule’s X-axis is defined as the vector from its oxygen atom to one of its hydrogen atoms. Its Z-axis is then defined as perpendicular to the X-axis and the HO-H plane, and its Y-axis is perpendicular to the X- and Z-axes. The line of nodes (vector N in Figure S1), is the line where the XY planes of the rotating and fixed frames of reference meet, and it is perpendicular to the Z-axes of both frames. The angle q is the angle between the two Z-axes, while f and y are the angles between the line of nodes and the fixed-frame X-axis and the water-frame X axis, respectively. Molecular dynamics methods Force-field parameters of the synthetic host molecule cucurbit[7]uril (CB7) (Error! Reference source not found.) were generated as follows. Partial charges were computed with the program RESP78, part of AMBER 1164, based on electronic structure calculations at the 6-31G* level with the program Gaussian 2003. The remaining parameters were assigned from the GAFF79 force field, with the AmberTools program Leap. The circular host molecule, which is about 13Å in diameter, was then computationally immersed in a 36Å x 39Å x 38Å box of preequilibrated TIP4PEW water molecules, using the program Leap. The resulting system consisted of 126 solute atoms and 1699 water molecules. This initial system was relaxed with 1500 cycles of steepest descent followed by 500 cycles of conjugate gradient energy-minimization. The minimized system was then heated to 300K in steps of 50K, each lasting 20ps. The system was then equilibrated for 5ns at 300K. For the equilibration simulations, constant 1 atm pressure was maintained with isotropic positional scaling and a relaxation time of 0.5 ps to ensure that the system density remained appropriate. A 400ns NVT production run was then carried out and trajectory frames for analysis were saved at 0.5 ps intervals. All simulations were carried out with a pre-release graphical processor unit-enabled version of the Amber 12 program PMEMD64,80,81 using Langevin dynamics82 at 300K with a collision frequency of 2 ps-1, periodic boundary conditions, a nonbonded cutoff distance of 8.0 Å coupled with Particle-Mesh Ewald long-ranged electrostatics83, a time step of 2fs, and SHAKE84 for covalent bonds to hydrogen atoms. Center-of-mass translation of the host was removed every 1000 steps to keep the system centered. Additional Figures Figure S2. Number of water molecules within the CB7 cavity as a function of simulation time. Only 50 ns of the longer simulation are plotted, so that transitions may be discerned. Figure S3. Probability density functions of Euler angles for waters in various voxels. Left: a low orientational entropy voxel near the carbonyl oxygens. Middle: a highly occupied voxel in the torus region for regular CB7. Right: a highly occupied voxel in the toroidal region of for nonpolar CB7. Note that, for one angle, the x-axis corresponds to cos(θ) instead of θ. All three distributions are flat for bulk water. Figure S4. Convergence with simulation time of normalized water properties, as labeled, for the four regions defined in text: Torus, Cavity, Torus Surface and Cavity Surface.