docx - STAO
advertisement

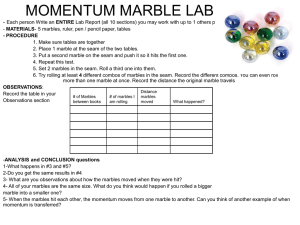
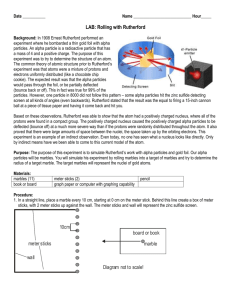
SNC1D/1P Atoms, Elements, and Compounds/Exploring Matter Student Activity: Rutherford’s Black Box Topics Timing atomic structure scientific method preparation: 1–1.5 h (first time only) demonstration: 15–20 min Specific Expectations SNC1D A1.5 conduct inquiries, controlling some variables, adapting or extending procedures as required, and using standard equipment and materials safely, accurately, and effectively, to collect observations and data A1.6 gather data from laboratory and other sources, and organize and record the data using appropriate formats, including tables, flow charts, graphs, and/or diagrams A1.10 draw conclusions based on inquiry results and research findings, and justify their conclusions C3.1 explain how different atomic models evolved as a result of experimental evidence (e.g., how the Thomson model of the atom changed as a result of the Rutherford gold-foil experiment) C3.2 describe the characteristics of neutrons, protons, and electrons, including charge, location and relative mass SNC1P A1.1 formulate scientific questions about observed relationships, ideas, problems, and/or issues, make predictions, and/or formulate hypotheses to focus inquiries or research A1.5 conduct inquiries, controlling some variables, adapting or extending procedures as required, and using standard equipment and materials safely, accurately, and effectively, to collect observations and data A1.6 gather data from laboratory and other sources, and organize and record the data using appropriate formats, including tables, flow charts, graphs, and/or diagrams A1.10 draw conclusions based on inquiry results and research findings, and justify their conclusions C3.1 identify the characteristics of neutrons, protons, and electrons, including charge, location, and relative mass Introduction We are most accustomed to making observations directly with our eyes. Students therefore often find it conceptually challenging to comprehend the reasoning and logic used by scientists studying atoms that they cannot directly see. This activity allows the students to practice making logical inferences in an experiment with very similar principles to that of Rutherford’s famous gold foil experiment. Materials 4 film canisters or wooden cylinders for each “black box” poster board, or ply wood cut into 30 cm × 30 cm squares 4 screws block of wood, about 5 cm across, cut into a unique shape (e.g., square, circle, diamond, or rectangle)screwdriver glue (white glue or wood glue) 2 or 3marbles Safety Considerations Take care when handling wood-working equipment. Use professional judgement with rowdy classes if students may be inclined to throw marbles. Procedure 1. 2. 3. 4. 5. Screw the caps of the film canisters to the poster board/plywood at the four corners, and then fasten the film canister bottoms to the lids. Glue the uniquely shaped block of wood to the underside of the board (Fig.1). Do not use screws because the students will be asked to determine where the “nucleus” is. Screws passing through the board will give it away. Predict/Explain Have students predict how the path of the marble will be affected by variously shaped wood blocks. Observe The students will take turns to roll or flick a marble under the poster board, and observe where it exits. The students will need to record data about entry and exit points of the marbles, as well as angles of deflection, and make logical conclusions about the shape of the “nucleus.” Explain Ask students to reconvene in groups and discuss why the marbles deflect in the patterns observed. They should try to make predictions, based on their observations, about the location and shape of the “nucleus.” Fig.1 Setup for activity Disposal Keep the “black boxes” for future use to save preparation time. What happens? In this model the marbles represent alpha particles interacting with the nucleus (the wooden block). The alpha particles’ movement will be affected by the nucleus, and deflected at different angles depending on the angle of incidence and the shape of the nucleus. How does it work? Students should be able to interpret the change in direction of the marbles and so draw conclusions about the size and shape of the hidden object (Fig.2). This is the technique that Rutherford used to develop his theory of atomic structure. Fig.2 The marbles will be reflected differently, depending on the size and shape of the object Teaching Suggestions/Hints 1. 2. 3. 4. Hardware and lumber stores sometimes cut wood to size for a small fee. This activity works best if the students are able to interact with it directly, so it should be a student activity rather than a demonstration. If you intend to ask the students to predict the shape of the wood block, first draw some examples on the board showing how they would expect the marbles to be deflected. To deter peeking, place the “black boxes” at the work stations before students arrive in the room. If you feel that your students will find making the predictions about nucleus shape too abstract, you could make this activity more concrete (and more messy) by placing a large sheet of white paper under the “black box” and dipping the marbles in water-based paint before rolling them. This will record the path of the marble, allowing the paper to be removed after the students have rolled about a dozen marbles so that they can get a clearer picture what was happening inside the box. An alternative exercise can be done by taking a small object such as a marble or a nail and completely covering it with modelling clay. Give the students toothpicks and the clay ball, and ask them to determine the shape of the hidden object just by probing the ball with the toothpick Next Steps Now that students have acquired some understanding of how the concept of the atomic nucleus was discovered, the next logical step is to explain the organization of electrons in the atom according to the Bohr–Rutherford model. Show students how to represent electrons using Bohr– Rutherford diagrams. Additional Resources 1. 2. This detailed description is provided of the “Rutherford Roller” lesson includes photos of the materials being assembled and diagrams explaining possible results of this simulation: http://www.exo.net/~emuller/activities/Rutherford%20Roller.pdf This video illustrates how Rutherford and his co-workers studied the angles at which alpha particles were scattered as they passed through a thin gold foil and helped them to develop the concept of an atomic nucleus: http://www.youtube.com/watch?v=5pZj0u_XMbc