Marijuana and its derivatives in the treatment of epilepsy
advertisement

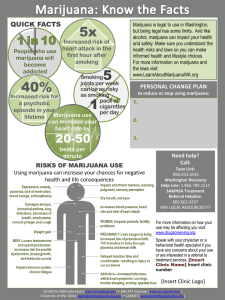
*`Marijuana and its derivatives in the treatment of epilepsy Patients, families, carers and doctors recognise the need, sometimes a desperate need, for additional and more effective therapies for epilepsy. Desperate times may on occasions justify desperate measures, but medical treatment decisions should always be based on knowledge of potential risks and benefits of the therapy. Anecdotal reports of patients experiencing dramatically beneficial responses to derivatives of marijuana, “medical marijuana”, has brought renewed attention to the potential for marijuana to as an antiepileptic therapy and provide further insights to the mechanisms of epilepsy1. It is important to note that there are also reports of people who experience worsening of seizure control with the use of marijuana. High quality evidence from randomised controlled trials from which to assess anti-epileptic efficacy of cannabinoids is scant.2 The pharmaceutical product, cannabidiol (Epidiolex™), has been given Orphan Drug status by the Food and Drug Agency of the United States as an investigational drug therapy for patients with Dravet and Lennox-Gastaut syndromes. An ongoing open label study recently released data for 27 patients treated for more than 12 weeks3. Four patients were seizure free and approximately half the patients had a 50% reduction in seizures. Somnolence, fatigue, diarrhoea and altered appetite were each seen in more than 10% of these patients. The adverse effects on health from marijuana intake include effects on brain development, risk of addiction, cognitive impairment during times of intoxication, subsequent psychiatric illness and increased risk of motor vehicle accidents4. There are major limitations regarding the knowledge of long term effects of marijuana intake and whether it is teratogenic. Further, the increase in the tetrahydrocannabinol concentration of marijuana in the United States is estimated to have risen from 3% to 12% over the last 30 years. This may lead to increased toxicity4. How should neurologists advise patients regarding “medical marijuana” for the treatment of epilepsy until there is there is sufficient, good quality evidence to allow informed decisions be best managed? Firstly, the extent of uncertainty regards efficacy and safety of marijuana derivatives should be explained to patients and families. Secondly, the patient or family should be encouraged to inform a treating doctor if this alternative therapy is being commenced. If a significant change in the patient’s health were to occur then this can be more completely assessed. Thirdly, definitive answers regarding efficacy and safety of marijuana to treat people with epilepsy needs to be ultimately obtained from properly constructed and ethically approved double-blind randomized placebo-controlled trials, in both paediatric and adult populations. Finally, individual doctors should determine their position on how the use of an illicit substance to obtain a therapeutic benefit should be appropriately managed. 1. The case for medical marijuana in epilepsy. Maa E, Figi P Epilepsia 2014;55:783-6 2. The case for assessing cannabidiol in epilepsy. Cilio MR, Thiele EA, Devinsky O. Epilepsia 2014;55:787-90 3. GW Pharmaceuticals announces physician reports of Epidiolex treatment effect in children and young adults with treatment-resistant epilepsy from physician-led expanded access treatment program. http://www.gwpharm.com/GW%20Pharmaceuticals%20Announces%20Physician%20Report s%20of%20Epidiolex%20Treatment%20Effect%20in%20Children%20and%20Young%20Adult s%20with%20Treatment-Resistant%20Epilepsy%20from%20PhysicianLed%20Expanded%20Access%20Treatment%20Program.aspx Accessed 1 September, 2014 4. Adverse Health Effects of Marijuana Use. Volkow ND et al. N Engl J Med 2014;370:2219-2227