eGFR-C BVS Laboratory Follow up Data Collection Form v1.0
advertisement

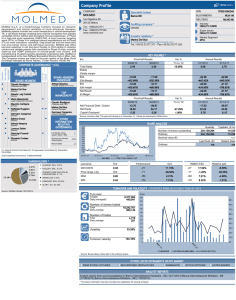
ISRCTN42955626 Confidential once completed Please answer all the questions eGFR-C Biological Variability Study Laboratory Data Collection Form (To be used for samples with Study Number starting Bxxxxx only) Assessment visit (please tick) 20 patient study of intra-individual biological variability (Canterbury only) iiLii Baseline iiLii Week 2 iiLii Week 3 iiLii Week 4 Part A: Identifying Details Study No.: ii I ii I ii I ii ii I ii CRF completed by: (Please PRINT): ……………………………………..……………… Date of completion: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Patient’s DOB: iMi Mi iMi / Yii iYii iYii iYii Part B: Analysis Results Code / Colour LSCr / Yellow Test Result Date Serum Creatinine (mass spectrometry) ANALYSIS 1 Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ii I ii I ii I i . i (μmol/L): LSCr / Yellow Serum Creatinine (mass spectrometry) ANALYSIS 2 ii I ii I ii I i . i (μmol/L): KSCy / Yellow Serum Creatinine (enzymatic) ANALYSIS 1 ii I ii I ii I i . i (μmol/L): KSCy / Yellow Serum Creatinine (enzymatic) ANALYSIS 2 ii I ii I ii I i . i (μmol/L): KSCy / Yellow KSCy / Yellow Cystatin C (mg/L): Cystatin C (mg/L): ANALYSIS 1 i I i .. ii I ii ANALYSIS 2 i I i .. ii I ii Please enter data online at https://www.trials.bham.ac.uk/egfrc or return form to: eGFR-C Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. eGFR-C – BVS Lab Data Collection Form Page 1 of 5 Version 1.0 14th Feb 2014 ISRCTN42955626 KUACR /Neutral KUACR /Neutral Confidential once completed Albumin Creatinine Ratio (ACR, mg/mmol): ANALYSIS 1 Albumin Creatinine Ratio (ACR, mg/mmol): ANALYSIS 2 ii I ii I ii I i ii I ii I ii I i . i . Please answer all the questions Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii i eGFR The Chief Investigator will be notified if any of the GFR estimates are <15 mL/min/1.73 m2. eGFR (mL/min/1.73 m2): MDRDcreatinine – ANALYSIS 1 I ii I ii I i . ii MDRDcreatinine – ANALYSIS 2 I ii I ii I i . ii CKD-EPIcreatinine – ANALYSIS 1 I ii I ii I i . ii CKD-EPIcreatinine – ANALYSIS 2 I ii I ii I i . ii CKD-EPIcystatin – ANALYSIS 1 I ii I ii I i . ii CKD-EPIcystatin – ANALYSIS 2 I ii I ii I i . ii CKD-EPIcystatin-creatinine – ANALYSIS 1 I ii I ii I i . ii CKD-EPIcystatin-creatinine – ANALYSIS 2 I ii I ii I i . ii mGFR (reference GFR measurement by iohexol) Code Sample time Iohexol (mmol/L) point (mins) ANALYSIS 1 KIH5 / Green KIH5 / Green 5 I ii I ii I i . ii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ANALYSIS 2 5 KIH120 120 I ii I ii I i ANALYSIS 1 . ii Please enter data online at https://www.trials.bham.ac.uk/egfrc or return form to: eGFR-C Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. eGFR-C – BVS Lab Data Collection Form Page 2 of 5 Version 1.0 14th Feb 2014 ISRCTN42955626 / Green Confidential once completed I ii I ii I i KIH120 120 / Green . ii KIH180 180 / Green I ii I ii I i . KIH240 240 / Green I ii I ii I i . ii LIH5 / Green 5 I ii I ii I i . LIH120 / Green I ii I ii I i . ii LIH180 / Green Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ANALYSIS 2 I ii I ii I i . ii I ii I ii I i . ii ANALYSIS 2 5 I ii I ii I i . ii 120 I ii I ii I i . ii ANALYSIS 2 120 I ii I ii I i . ii ANALYSIS 1 LIH180 / Green iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ii ANALYSIS 1 LIH120 / Green Date of Analysis: ANALYSIS 2 ANALYSIS 1 LIH5 / Green iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ii ANALYSIS 1 KIH240 240 / Green Date Received: ANALYSIS 2 ANALYSIS 1 KIH180 180 / Green Please answer all the questions 180 180 I ii I ii I i . ii ANALYSIS 2 Please enter data online at https://www.trials.bham.ac.uk/egfrc or return form to: eGFR-C Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. eGFR-C – BVS Lab Data Collection Form Page 3 of 5 Version 1.0 14th Feb 2014 ISRCTN42955626 Confidential once completed I ii I ii I i . ii ANALYSIS 1 LIH240 / Green LIH240 / Green I ii I ii I i 240 . Please answer all the questions ii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii ANALYSIS 2 240 I ii I ii I i . ii KENT USE ONLY: GFR (mL/min/1.73 m2): The Chief Investigator will be notified if the value is <15 mL/min/1.73 m2. ANALYSIS 1 I ii I ii I i . ii . ii ANALYSIS 2 I ii I ii I i LONDON USE ONLY: GFR (mL/min/1.73 m2): The Chief Investigator will be notified if the value is <15 mL/min/1.73 m2. ANALYSIS 1 I ii I ii I i . ii . ii ANALYSIS 2 I ii I ii I i Part C: Biomarkers Sample Code Sample KUBM / Neutral (first sample) Urine: KUBM / Neutral (second Urine: Date Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Please enter data online at https://www.trials.bham.ac.uk/egfrc or return form to: eGFR-C Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. eGFR-C – BVS Lab Data Collection Form Page 4 of 5 Version 1.0 14th Feb 2014 ISRCTN42955626 Confidential once completed sample) LUBM / Neutral KPBM / Purple LPBM / Purple KSBM / Yellow LSBM / Yellow Urine: Plasma: Plasma: Serum: Serum: Please answer all the questions Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Sample taken: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date Received: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Date of Analysis: iDi iDi / Mi iMi iMi / Yii iYii iYii iYii Thank you for completing the eGFR-C Laboratory Data Collection Form. Please enter data online: eGFR-C Online Data Entry: https://www.trials.bham.ac.uk/egfrc (24hrs) or return to: eGFR-C Study Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham, B15 2TT Tel: 0121 415 9130 Fax: 0121 415 9135 eGFR-C Study Website: http://www.birmingham.ac.uk/egfrc eGFR-C Study Mailbox: eGFR-C@trials.bham.ac.uk Please enter data online at https://www.trials.bham.ac.uk/egfrc or return form to: eGFR-C Office, Birmingham Clinical Trials Unit, College of Medical & Dental Sciences, Robert Aitken Institute, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. eGFR-C – BVS Lab Data Collection Form Page 5 of 5 Version 1.0 14th Feb 2014