The Retinopathy Of Prematurity Screening and Risk Assessment Tool
advertisement

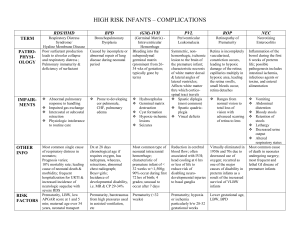
Running Head: THE RETINOPATHY OF PREMATURITY SCREENING AND RISK ASSESSMENT TOOL The Retinopathy Of Prematurity Screening and Risk Assessment Tool (ROPSRAT) Clinical Decision Support System 406-DL CDSS Northwestern University Submitted to: Professor Robert Cundick Submitted by Christopher Kelly and Maria Almacen March 2014 The Retinopathy of Prematurity Risk Assessment Tool 1 Abstract Retinopathy of prematurity (ROP) is a potentially blinding disease affecting premature infants. It is critical that premature babies be screened and treated in an appropriate fashion. The Retinopathy of Prematurity Screening and Risk Assessment Tool (ROPSRAT) is a clinical decision support tool (CDSS) designed for use with the Epic electronic medical record (EMR) program with a two-fold purpose: It implements the latest clinical recommendations for screening and provides a quantitative risk estimate to facilitate communication between the ophthalmologist and Neonatal Intensive Care Unit (NICU) staff, as well as between Neonatal Intensive Care Unit (NICU) staff and the families of premature infants. Data gathered by ROPSRAT can be used to evaluate ophthalmologists and NICUs to improve outcomes. ROPSRAT uses the terminology of the International Classification of Retinopathy of Prematurity (ICROP) to apply the latest clinical guidelines. This paper discusses the goals and design of ROPSRAT, along with stakeholders and its effect on workflow. It then presents a change management plan to facilitate its introduction into the NICU along with a discussion of content specification, user interface, and method of evaluation. Keywords: retinopathy of prematurity, screening guidelines, clinical decision-support system. The Retinopathy of Prematurity Risk Assessment Tool 2 Table of Contents Introduction ..................................................................................................................................... 4 Goals ............................................................................................................................................... 5 Intervention Selection for ROPSRAT ............................................................................................ 7 Objective 1: Develop a recommendation tool on the appropriate timing for follow-up and treatment of ROP that is accurate and timely integrated in clinician’s workflow based on the most current guidelines set by the AAP. ..................................................................................... 7 Objective 2: To increase family satisfaction by use of a developed tool that quantifies the risk of an infant’s ROP progression to be utilized by NICU staff or ophthalmologists for communication with families. ..................................................................................................... 7 Objective 3: To decrease incidence of critical ROP or incidence of blindness among premature infants by using a tool that allows quantitative risk comparison across NICUs. ....... 9 Objective 4: To increase rate of consistencies in among ophthalmologists by the use of a tool that allows comparison of ophthalmologist’s performance or practice. ................................... 12 Information System Inventory ...................................................................................................... 14 Information Sources ...................................................................................................................... 15 User Input...................................................................................................................................... 16 Output ........................................................................................................................................... 16 Stakeholders .................................................................................................................................. 17 Change Management Plan ............................................................................................................ 20 Treatment and Follow-up Recommendations ........................................................................... 21 Risk Score Calculation .............................................................................................................. 22 Planning and Development. ................................................................................................... 24 Post Implementation. ............................................................................................................. 26 Evaluation ..................................................................................................................................... 26 System Response Time ............................................................................................................. 27 Structure .................................................................................................................................... 27 Process ....................................................................................................................................... 27 Outcome .................................................................................................................................... 29 Discussion ..................................................................................................................................... 30 References ..................................................................................................................................... 32 Table ............................................................................................................................................. 34 The Retinopathy of Prematurity Risk Assessment Tool 3 Figures........................................................................................................................................... 35 Figure 1 ..................................................................................................................................... 35 Goals and Objectives ................................................................................................................ 35 Figure 2 ..................................................................................................................................... 36 Pop-up screen showing recommended follow up. ..................................................................... 36 Figure 3 ..................................................................................................................................... 37 Sample letter to families of infants with stage 2/zone 2/no plus ROP and 25% calculated risk of progression to needing laser surgery. ................................................................................... 37 Figure 4 ..................................................................................................................................... 38 Stakeholder Workflow for the Follow-up Recommendation Tool and Order set ...................... 38 The Retinopathy of Prematurity Risk Assessment Tool 4 Introduction ROP is a potentially blinding disease that affects the eyes of premature infants. Normally, an infant’s retina does not become fully vascularized until shortly before birth. When infants are born prematurely, the normal vascularization process can be altered. Instead of blood vessels growing out from the back of the eye, along its inside and towards the front, blood vessel growth stops. As the infant matures, vessel growth resumes but can do so in a pathologic way. The vessels may grow inwards, towards the center of the eye. When this happens, they can end up tearing the retina off the eye, bringing about total retinal detachment and complete blindness. Fortunately, a treatment exists. Most blindness can be prevented if the disease is identified and treated early. A major step in developing a treatment was the creation of a consistent terminology for the condition. The ICROP was initially published in 1984 and most recently revised in 2005. It represents the collaborative efforts of specialists across the world (International Committee for the Classification of Retinopathy of Prematurity, 2005). The ICROP divided the infant’s retinas into three zones. The disease was then labeled according to zone involvement. Zone 1 disease, closest to the back of the eye, occurs when blood vessel development is most severely altered. Zone 3 disease, closest to the front, is typically mild in form. In addition to zones, there are six stages, ranging from Stage 0, where the vessels are immature and gradually taper off, to Stage 5, where the retina has completely detached and the child is permanently blind. Finally, there are terms used to describe the appearance of the retinal blood vessels. “Plus disease” describes a significant amount of retinal blood vessel dilation and tortuosity and is a poor prognostic indicator. Less severe is “pre-plus disease.” “No plus” is used when the blood vessels appear normal. The Retinopathy of Prematurity Risk Assessment Tool 5 Several large-scale clinical trials have shown that treatment can be effective in preventing the progression to Stage 5 if performed at the appropriate time, so it becomes critical that these infants have retinal examinations before they get to this point. Clinical guidelines have been published recommending appropriate times for screening and follow up based on the infant’s post conceptual age at birth, birth weight, current zone, and current stage. The most recent clinical guideline was published in 2013 (American Academy of Pediatrics, Section on Ophthalmology, et al., 2013). Infants with stage 1, 2, or 3 may need to have laser surgery depending on the zone and blood vessel appearance to prevent the progression to stage 4, a partial retinal detachment, or stage 5, complete blindness. Goals Although the incidence of blindness is about ten times lower in children compared to adults, retinopathy of prematurity is a disease that afflicts many premature infants due to advances in neonatal care (“Facts about retinopathy,” n.d.). As more premature infants are being saved the incidence of ROP increases. The more premature the age of the infant the higher risk for ROP. While approximately 16,000 infants develop ROP by some degree, almost 1500 infants in the United States progress with ROP severe enough to require medical or surgical treatment for the disease, and about 600 of these infants become legally blind each year (“Facts about retinopathy,” n.d.). The financial cost of managing a blind or visually impaired child is enormous and the disruption of the child’s and family’s life is incalculable. This financial burden is also shared by society and healthcare payer in providing for the medical or surgical need and home care support of a disabled child. In contrast to the slowing economy, health care expenditure grew faster from $1106 per individual to $6280 in 24 years from 1980 (Stanton, 2006). With the accelerating pace of medical innovation and aging population combined, health The Retinopathy of Prematurity Risk Assessment Tool 6 care costs will continue to rise from 9 percent to more than 16 percent (Stanton, 2006). Although complete blindness from ROP fortunately is a relatively rare condition, the cost to society of a blind infant is enormous. Malpractice judgments reflect this. Plaintiffs have been awarded over $10 million in court cases (Day, Menke, & Abbott, 2009). ROP is thus an important condition from a societal standpoint, as well as for healthcare organizations that take care of premature infants. Improving the care, as well as communication with families and family satisfaction, has the potential to mitigate significant financial risk. It is, therefore, imperative for health care providers to work together in providing better health care quality at a reduced cost. Our organization aims to improve the visual health among premature infants and optimize cost-effectiveness of visual care (see Figure 1). Clinically, our goal is to improve the quality of ROP care and enhance communication of the condition among clinicians and families For these reasons, our institution intends to develop the Retinopathy of Prematurity Screening and Risk Assessment Tool (ROPSRAT). This ROPSRAT project aims to achieve the following objectives (also see Figure 1): 1. Develop a recommendation tool on the appropriate timing for follow-up and treatment of ROP that is accurate and timely integrated in clinician’s workflow based on the most current guidelines set by the AAP. 2. To increase family satisfaction by use of a developed tool that quantifies the risk of an infant’s ROP progression to be utilized by NICU staff or ophthalmologists for communication with families. 3. To decrease incidence of critical ROP or incidence of blindness among premature infants by using a tool that allows quantitative risk comparison across NICUs. The Retinopathy of Prematurity Risk Assessment Tool 7 4. To increase rate of consistencies among ophthalmologists by the use of a tool that allows comparison of ophthalmologist’s performance or practice. Intervention Selection for ROPSRAT Objective 1: Develop a recommendation tool on the appropriate timing for follow-up and treatment of ROP that is accurate and timely integrated in clinician’s workflow based on the most current guidelines set by the AAP. As a mechanism for improving the quality of visual care of premature infants, the Retinopathy of Prematurity Screening and Risk Assessment Tool (ROPSRAT) will function as a decision support tool in two ways: as a clinical decision support tool and risk assessment tool. As a decision support tool, it will implement the most recent ROP screening guidelines. The tool will be updated every few years to take into account advances in the diagnosis and treatment of ROP. Screening for ROP is typically done by pediatric ophthalmologists in larger NICUs. However, in smaller NICUs, screening may be done by general ophthalmologists who may not be aware of the latest recommendations. Treatment is often done by retinal specialists and typically involves laser surgery. Although the indications are very different, the operation is technically similar to the laser surgery done for proliferative diabetic retinopathy. While ROP screening may involve five percent or more of a pediatric ophthalmologist’s practice, it is typically less than one percent of the retinal specialist’s, who also may not be as aware of the latest recommendations. The screening component of ROPSRAT provides an alert to ophthalmologists recommending appropriate follow up based on the latest eye examination findings. For instance, once the ophthalmologist enters the exam results of “Stage 1/Zone 2/No Plus”, an alert box will The Retinopathy of Prematurity Risk Assessment Tool 8 pop up recommending a follow-up eye examination in two weeks, as per guidelines published by the American Academy of Pediatrics (2013). Figure 2 shows a mock-up of how this warning box will appear. Clicking the “Follow up in 2 weeks” button will trigger an Epic “SmartSet” order entering the infant’s name into a list of infants who will be seen in two weeks. The warning box also allows an override for when the examining ophthalmologist feels that an examination should be sooner or later than two weeks. In this case, a calendar will pop up and the ophthalmologist will schedule follow up by clicking the appropriate date. There will be several alternative screens, saying “Surgery is recommended,” “Follow up in 1 week,” “Follow up in 2 weeks,” or “Follow up in 6 months.” Scheduled follow up visits will be stored in a system-wide electronic database. The most recent guidelines will be incorporated into the alerts, making the right thing to do as the easy thing to do. The alert message will also show if surgery is indicated. Once the risk of blindness no longer exists (the retina is “mature” or the ROP has regressed), an alert will pop up saying follow-up is recommended in six months as these infants are at a higher risk of amblyopia and strabismus. This intervention has the advantage of taking place immediately after the exam, while the data is being entered into the computer by the ophthalmologist, and so will have minimal effect on current workflow. The alerts can be configured into an Epic “SmartForm” using a series of “If…Then” statements. There are three zones and four stages that occur prior to the retinal detachment stages. Additionally, there are three terms to describe the appearance of the retinal vessels. Counting “regressed” and “mature” descriptions, there are 38 possible combinations. It may be difficult even for an experienced pediatric ophthalmologist to keep track all these, especially in light of changing guidelines. The Decision Logic table shows all these possibilities and the The Retinopathy of Prematurity Risk Assessment Tool 9 corresponding recommendations according to the 2013 American Academy of Pediatrics guidelines. As guidelines change in the future, it should be a fairly straightforward process to modify the system’s responses once they are configured in the system. If additional descriptive terms are added (for example, if a term is added for “posterior zone 2”; see discussion below), it will be possible to add those terms to the decision logic as well. Objective 2: To increase family satisfaction by use of a developed tool that quantifies the risk of an infant’s ROP progression to be utilized by NICU staff or ophthalmologists for communication with families. ROPSRAT Risk Assessment, the second portion of the clinical decision support tool, is intended to improve communication between screening ophthalmologists and NICU staff, along with the parents of the infants at risk. It will also provide a mechanism for measuring and comparing outcomes between NICUs and the evaluation of ophthalmologists. Several risk factors have been identified that influence the risk of an infant progressing to the point where treatment is indicated, the most important being post-conceptual age at birth and birth weight. There are other influences as well, including infection and other comorbid conditions, but these are not consistently identified in retrospective analyses. By incorporating post conceptual age at birth, birth weight, current age, stage, zone and a measure of blood vessel appearance into a model, an estimate can be made of the risk of progression to needing laser surgery. This estimate can then be conveyed to neonatologists, neonatal nurses, and the infant’s parents. Developing a risk assessment model may be more problematic. There are a number of risk factors that determine the probability of progression to the point where treatment is needed. Well known risk factors include birth weight, post conceptual age of birth, current age, current zone, stage and blood vessel appearance. Other risk factors have been identified, but they may The Retinopathy of Prematurity Risk Assessment Tool 10 be more dependent on local factors since they do not seem to consistently show up in retrospective analyses. Multivariate regression analysis will be used to create a model that predicts each child’s risk. Each of the variables that are known to influence progression can be given a weight according to historical data. Once sufficient data has accumulated, values for each baby’s risk factors can be used to calculate the risk of progression after each exam. However, even a multivariate regression analysis would not necessarily be simple. Infants born at 23, 24, and 25 weeks are at significantly higher risk than infants born at 28 weeks, and risk may decline more quickly than a linear model might predict. Additionally, current age influences the likelihood of progression to needing laser surgery. However, the most common age for needing laser surgery is 35 to 36 weeks post conceptual age. After that, even for infants with the same stage, zone, and blood vessel appearance, the risk begins to decline. Any meaningful multivariate regression analysis will need to include nonlinear parameters in its calculation of risk score. Alternatively, statistical techniques using a survival curve may be beneficial. This may need to be explored in more detail. Once the score is calculated after each exam, it could then be made part of the patient’s record so NICU staff can follow and monitor from week to week whether the score, and consequently the infant’s risk, is increasing or decreasing. It would serve to alert neonatologists as to which neonates face the highest risks of progression. Such alerts may influence decisions about discharge or transfer. One important consideration is that eye disease does not occur in isolation; these babies often have other co-morbid conditions, and a probability score could be helpful in scheduling other procedures. For example, it might be better to delay a hernia repair on a child if eye surgery is imminent. The Retinopathy of Prematurity Risk Assessment Tool 11 Having a premature, ill, and hospitalized infant can be a terrifying experience for parents. So many systems can be affected and so many critical health issues can develop, that parents easily become overwhelmed. The idea that an infant could end up blind may be a low priority when the infant is unable to breathe. On the other hand, being told about a risk of blindness could be terrifying for parents, even in cases where the risk is low. One difficulty exists in healthcare is that qualitative terms expressing risk like “unlikely” or “possible” may be interpreted differently by different people. Parents who are told that their child may go blind may over-interpret the risk of such a severe event. As discussed in Hunink, Glasziou & Siegel, et al. (2001, p. 34-35), there are advantages to being able to express uncertainty and risk quantitatively. A quantified risk score can help focus parental concern on salient risks. In addition to the NICU staff, the score will also be given to parents each week to help them understand their preemie’s risk. It is likely that interpreting such a score would not be an easy task for most parents, so it will be critical to have neonatal nurses understand the risk and be able to translate this score for parents. Preparing parents for possible laser surgery several weeks in advance could be a major step in improving family satisfaction. A pre-exam risk score will be based on birth weight and post conceptual age at birth. After each exam, a calculation will be performed by the Epic “Smart Form” using results from a recent multivariate regression analysis. This score will then be incorporated into the medical record for neonatologists. The score will also be included in an information sheet for the family. Infants at high risk will get different information than infants at low risk. A sample parental information sheet for a baby with stage 2/zone 2/no plus disease and a calculated 25% chance of progression is shown in Figure 3. This letter will be auto-generated by Epic with links showing the infant’s name, gender, and risk, as well the name of the ophthalmologist. Different letters The Retinopathy of Prematurity Risk Assessment Tool 12 will be generated for infants at LOW, MODERATE, and HIGH risk, and for infants requiring surgery. When an infant’s eyes are no longer at risk for blindness, a different letter is generated discussing that infants born prematurely have a higher risk of developing amblyopia, strabismus, and corrective glasses at a young age, and recommending a follow-up in six months. The letter may also be translated into Spanish, and other languages as needed. After each exam, parents will see if their child’s disease is progressing based on whether the score increases or decreases. As the score increases, the neonatologist and NICU staff will discuss the risks with an increasing sense of urgency utilizing the information generated by the ROPSRAT CDSS. Objective 3: To decrease incidence of critical ROP or incidence of blindness among premature infants by using a tool that allows quantitative risk comparison across NICUs. Differences in populations being compared can make assessing outcomes in different geographic locations challenging. Providing a quantitative risk analysis will allow the ROPSRAT compare outcomes in different NICUs in consideration of risk adjustment. A multivariate non-linear regression model will be used by ROPSRAT to evaluate quality. While it is possible to determine the number of infants at each NICU who progress to the point of needing surgery, an absolute proportion without risk adjustment is largely meaningless. However, with ROPSRAT, it will be possible to determine if an infant born at one NICU has a higher risk for progression than a baby with similar characteristics born in another. Does a baby born at 25 weeks and 650 grams, now at 33 weeks and having stage 2/zone 2/no plus, for example, have a higher chance of progressing in one NICU than another? It will likely take years of data to develop sufficient statistical data analysis to answer this question, but if results are not quantified, improvements cannot be made. Such information will allow data The Retinopathy of Prematurity Risk Assessment Tool 13 mining techniques to potentially determine other risk factors. It may turn out that local practices in care or monitoring could have an influence on outcomes that could not be predicted in advance. Objective 4: To increase rate of consistencies in among ophthalmologists by the use of a tool that allows comparison of ophthalmologist’s performance or practice. Similarly, evaluating physician’s eye examination techniques in varied settings can be particularly difficult, since the examination is only performed once each visit. ROPSRAT will allow risk-adjusted comparisons between ophthalmologists to see if there are any outliers in the application of specific definitions. With the ultimate goal of preventing vision impairment among infants, the ROPSRAT aims to manage this condition by allowing risk-adjusted comparisons between retina specialists that may identify those with worse outcomes. The ROPSRAT multivariate regression analysis model will also allow comparison among ophthalmologists as well. Three ophthalmologists employed by Connecticut Children’s Medical Center together perform approximately 1000 screening examinations each year. Since each exam is often performed by only one ophthalmologist, limited opportunities exist to make sure all ophthalmologists apply the standards in the same way. Zone, in particular, is challenging to ascertain since it can be difficult to determine locations within the retina. Quantified data could help confirm if an ophthalmologist is an outlier in his or her application of terms. For example, does one ophthalmologist significantly identify more zone 3 disease than would be predicted based on the characteristics of the infants examined? Additionally, one ophthalmologist may be an outlier in terms of his or her application of the current clinical guidelines, scheduling follow ups more or less often than recommended. This could certainly be illuminating, although it must The Retinopathy of Prematurity Risk Assessment Tool 14 be emphasized to ophthalmologists that the tool is not intended to “grade” them, rather to provide feedback that does not currently exist. What really matters is the long-term visual outcome of these children. Even if complete blindness does not result, delay in treatment can cause damage to the retina that prevents the child from reaching full visual potential. Laser surgery on these infants is technically challenging and some retinal specialists may be more proficient than others. Data collected by the ROPSRAT will allow risk-adjusted outcome comparisons. If outcomes are not measured, they cannot be improved. Information System Inventory The ROPSRAT will require a robust technology infrastructure for its success. It will be developed for use with the Epic EMR, taking advantage of the clinical decision support tools available in its ecosystem. However, since Epic EMR is already “live” in production prior to the initiation of the CDS intervention, separate hardware and software resources will not be necessary. As a result, performance, availability, security, and other technical as well as operational requirements for the ROPSRAT will be based on the requirements of the currently deployed Epic EMR. The ophthalmologist will enter data at bedside using a laptop or mobile device equipped with an Epic EMR client and connected to the network through a wireless access point. There are multiple workstations throughout the NICU for neonatologists and nurses to access the system, although there is no individual Epic client device at each bedside. Standard vocabularies have an important role in building ROPSRAT as it is essential for the attainment of semantic interoperability with other systems, such as for other provider’s The Retinopathy of Prematurity Risk Assessment Tool 15 adoption of the ROPSRAT CDSS or for government reporting purposes as recommended by Osheroff et al. (2012). The appropriate ontology for ROPSRAT may prove to be challenging. In ICD-9, there are codes that describe the stage of the ROP disease and a general term for “Retinopathy of prematurity, unspecified.” ICD-10 adds some specificity and includes codes for each stage, specifying right eye, left eye, both eyes, and unspecified eye. But even ICD-10 codes do not consider zone or blood vessel appearance, key elements in determining whether treatment is needed (ICD-10 Data.com, 2014). Fortunately, more detail is available within the database commonly used by Epic, so that the creation of additional diagnostic elements is not imperative. Even the Epic database may prove limiting however. In some situations it might be ideal to subdivide the categories further. Zones described by the ICROP scheme were based on landmarks within the retina to provide observer agreement. Not enough was known about the disease in 1984 to know if they reflected clinical reality. There is some evidence that disease occurring in posterior zone 2 may behave more like disease in zone 1 than disease in mid-or anterior zone 2 (Early Treatment for Retinopathy of Prematurity Cooperative Group, 2003). A category for “posterior zone 2” could be developed and applied locally. It might be valuable in retrospective evaluations and it is even possible that screening recommendations might vary for disease in this location. However, it would likely be mapped to the same zone 2 term in the Epic database. Information Sources The American Academy of Pediatrics updates clinical guidelines every few years, most recently in 2013 (American Academy of Pediatrics Section on Ophthalmology, et al., 2013). These are widely available. The Retinopathy of Prematurity Risk Assessment Tool 16 After ROPSRAT has collected sufficient data, data mining techniques can help determine other risk factors. A number of risk factors have been identified that potentially influence the risk of progression besides post conceptual age at birth and birth weight included in the ROPSRAT model. These factors range from ambient light exposure, to gender, to whether the infant is fed breast milk. However, these do not seem consistent across NICUs. Searching the literature will provide potential risk factors that can be used in a supervised data mining approach to determine which, if any, risk factors influence outcomes locally. Alternatively, there may be risk factors that have not been considered, demography for example, which a supervised data mining approach could use. The approach should be supervised, rather than unsupervised, since the progression of retinopathy is a predefined dependent variable (Hardin & Chhieng, 2007, p. 47). User Input The ophthalmologist will enter data on stage, zone, and blood vessel appearance into an Epic EMR SmartForm with decision logic built in. Other data, including birth data, gestational age at birth, and other health issues will be entered as part of the EMR. Output ROPSRAT will provide two types of output. First is an alert reminder for the ophthalmologist that will provide a recommendation for follow up or treatment. The second is a quantitative risk score for those infants that do not yet require treatment, ranging from near zero when infants with similar risk factors have never gone on to need surgery, to near 100% if progression is imminent. These risk scores would need to be expressed as a range, reflecting statistical uncertainty. The Retinopathy of Prematurity Risk Assessment Tool 17 Stakeholders ROPSRAT will need the support of, and will benefit from, individuals at multiple levels within the organization. The following are the stakeholders of this CDSS project: 1. Lead Clinician The lead clinician will be a pediatric ophthalmologist. The pediatric ophthalmologist is the expert in following and implementing the 2013 ROP guidelines that will be used in developing this CDSS. The lead clinician will be the main user of the CDSS, entering the results of the eye exams in the system and receiving the alert messages. This strategy will make ROPSRAT user-friendly and will ensure that it functions as useful and relevant as possible. The lead clinician, who is also the spokesperson for other pediatric ophthalmologists using the system, will work with the technical team and will communicate with staff about the interventions before, during, and after the launch. 2. Physician champion The physician champion will be a neonatologist. Neonatologists bear primary responsibility for the overall care of the neonate. They order the initial exam and often serve to communicate the ophthalmologist’s findings to the parents, since the parents may not be available when the eye exams are performed. Often, neonates are discharged or transferred when still at risk for eye disease progression. This makes neonatologists indispensable in ensuring appropriate follow up. The physician champion will provide essential input on how ROPSRAT will be incorporated into the EMR, and how the data will be accessed. The physician champion will also encourage discussions among other staff to address any concerns or issues. It will be the physician champion who is responsible for making the user interface as straight-forward for the The Retinopathy of Prematurity Risk Assessment Tool 18 NICU staff as possible. Ultimately, it is the physician champion who will manage and address the staff’s expectations. 3. Nurse Champion Nurses are on the front lines in the relationship between the NICU and the parents. Having a baby can be stressful for any family; having a sick preemie can be overwhelming. Parents who have discussions with ophthalmologists or neonatologists may not fully understand the implications of clinical information or may not come up with questions until later. This may be particularly true for ROP, since a problem that takes place inside the eyes may seem much less urgent than respiratory or infectious complications. The nurse champion must be a good communicator with years of experience as a neonatal nurse working with the neonatal population. It is critical that the nurse champion understands the needs of parents. What questions do parents typically ask? Where do misunderstandings commonly occur? The nurse champion must also be aware of the needs of fellow nurses so that information about ROP, as well as risks and follow up, is presented in a clear and unambiguous way. The nurse champion shall act as the spokesperson for the nurse end-users and handle the management of revisions and updates that will be necessary during and after the launch 4. Technical Specialist A technical specialist shall be tasked to ensure that the ROPSRAT implementation is properly deployed and correctly functions as intended. Since the EMR system will be Epic, the technical specialist must be an Epic clinical analyst. 5. Super Users The super users must be identified and be actively involved from inception. They must represent the main users of ROPSRAT, namely: a neonatologist, a pediatric ophthalmologist, and The Retinopathy of Prematurity Risk Assessment Tool 19 a neonatal nurse. It is critical for those who were not directly involved in the project development to have someone they can turn to for questions. 6. Chief Financial Officer and Chief Executive Officer Involvement of the higher levels of management will be required to finance and develop ROPSRAT and to align the project with the organization's overall goals. 7. Chief Medical Information Officer The Chief Medical Information Officer (CMIO), who reports to the Chief Information Officer and the Chief Medical Officer, shall be responsible for the first level approval of ROPSRAT prior to its development and shall also work to facilitate the approval and allocation of development funding. The CMIO must also see to it that the guidelines implemented represent the latest recommendations of American Association of Pediatrics. The CMIO shall present and recommend the CDSS project to the CIO, CMO, CFO, and CEO for further discussions and final approval for development. 8. Quality Officer The Quality Officer shall ensure that the development and implementation of the CDSS will uphold data quality improvement measures. The Quality Assurance Officer may help recognize potential opportunities and shall identify risks associated with the CDSS during development, as early identification of potential risks may help reduce actual risks. 9. Board of trustees Discussion with the members of the Board of Trustees will help clarify and highlight the role of the CDSS in addressing quality issues and in keeping the project in line with the organization’s overall goals and objectives. 10. EMR committee representative The Retinopathy of Prematurity Risk Assessment Tool 20 The EMR vendor (Epic) shall designate a committee representative who will provide guidance in integrating ROPSRAT into the EMR. This committee member shall bring relevant experience and invaluable input to CDSS development and deployment. 11. Risk Management Officer or Legal Counsel The Risk Management Officer or Legal Counsel shall determine the regulatory or legal risks involved in the development and deployment of ROPSRAT. One particular concern is that the risk of an infant’s disease progression may vary among institutions. If infants in a particular NICU are at a higher risk, is the organization exposed to any additional liability? Malpractice judgments for ROP can be in the tens of millions of dollars, so this condition is particularly fraught with financial and legal risks. In addition, communication with families must convey the risks of the condition in an appropriate way. 12. Patient Representatives Patient representatives are usually parents of NICU graduates. They can provide essential insights as to what families go through in this difficult setting. Their input could be invaluable in determining how relevant information is provided to families in an appropriate but not overwhelming manner. Their voice will provide critical input and will have a significant impact on the successful development and deployment of ROPSRAT. Change Management Plan The ROPSRAT CDSS is a two part project, providing a recommendation for follow up or treatment of infants for retinopathy of prematurity based on the latest clinical guidelines and serving as a communication mechanism between consultant ophthalmologists and NICU staff, and between NICU staff and patient families. Change management of each component will be handled separately. The Retinopathy of Prematurity Risk Assessment Tool 21 Treatment and Follow-up Recommendations Introducing ROPSRAT into the organization is the concern and responsibility of the project team leader who must understand the needs and concerns of the treating ophthalmologist stakeholders. The system is designed to improve their workflow and to make it easier to implement the latest recommendations. Some potential reasons for resistance to change include the fact that ROPSRAT may be perceived as threatening because it takes away physician autonomy. It is critical to make certain that ophthalmologists understand that these are recommendations, and that they have the ability to override them as they see clinically necessary. These recommendations must appear in a timely fashion. If there is a significant delay after the ophthalmologist enters his examination notes into the EMR, the ophthalmologist may become frustrated by the tool and may just proceed without the recommendation. To mitigate the risk of non-acceptance, the project team leader must act as a facilitator. ROPSRAT should not be imposed upon the users and the team leader must step back and allow the stakeholders to take part in the building process of the CDSS. The ophthalmology stakeholders must understand that this is being developed for them. Without doubt, there will be modifications after the roll out; this needs to be discussed in advance so stakeholder’s initial expectation is not perfection. The lead clinician will take the role of super-user and act as a liaison between users and the project team to help work out difficulties in user acceptance testing before it is offered more widely. This ophthalmologist’s role is vital in identifying errors in the offered recommendations. If the tool can be presented in such a way that ophthalmologists understand its use and purpose, and recommendations are made in a timely fashion, acceptance hopefully will not be a major concern. The Retinopathy of Prematurity Risk Assessment Tool 22 Risk Score Calculation ROP is a potentially blinding disease for premature infants, but only a small portion, less than 5%, of those infants who are screened for the disease require laser surgery for the condition, and only a small percentage of this population develops blindness despite laser surgery. The real success of ROPSRAT will be in how well it is accepted by the NICU staff and families. Generating a risk score will be meaningless if it is not used for management decisions or family discussions. Change management can be approached by the creation of a change management team. The change management team will be composed of a subsection of the project team, including an ophthalmologist, a neonatologist, and a NICU nurse. The impression of ROPSRAT’s designers is that ROP is often not well understood by the NICU staff, and that when a patient needs surgery, families are often taken unawares by a problem they did not realize was a major concern. Part of this stems from the fact that the ophthalmologists often round early in the morning when families are not present and so there is little opportunity for discussion. This has the potential to lead to misunderstandings, anger and ultimately blame when there is a bad outcome. ROP is a major malpractice concern (Day, Menke & Abbott, 2009). Judgments can be in the tens of millions of dollars. Improving communications can help reduce the risk of malpractice lawsuits. While it may be difficult to calculate a return on investment for this project based on financial considerations for very rare events, decreasing the risk of severe malpractice lawsuits will prove sufficient financial motivation. At the outset, the project leader must identify this issue and present it to the NICU staff, stressing the significance of communication to obtain parental satisfaction in reducing malpractice risk. However, prior to discussing it with the NICU staff, it might be worthwhile to The Retinopathy of Prematurity Risk Assessment Tool 23 gather two types of data. First, it would be useful to assess the understanding of ROP among the NICU staff. It could be presented as a survey or questionnaire. There have been a lot of changes in the understanding and management of this condition in the last two decades and their current knowledge may not reflect this. Likewise, some structured interviews with NICU staff could be performed. It may be informative to have the lead nurse on the project team observe a discussion with a nurse and a willing family about ROP risk. In addition to assessing staff understanding, there needs to be a means of assessing parental appreciation for the disease’s potential. It might be possible to take a survey of families who have “graduated” from the NICU, asking them about their experiences with retinopathy. Retrospective surveys may be difficult to interpret, so an alternative brief questionnaire could be administered by a discharge coordinator to randomly selected families. This information could be enlightening when presented to the NICU staff. It may be that parents have an adequate understanding of the condition and its consequences, but again, it is the impression of the developers of ROPSRAT that this is not the case. ROPSRAT will be initially developed for use at Connecticut Children’s Medical Center in Hartford, Connecticut. The structure of this institution presents some interesting opportunities. Connecticut Children’s manages two NICU’s, one at Hartford Hospital and one several towns away at the University of Connecticut. ROPSRAT could initially be rolled out at one institution and parental understanding and satisfaction with their child’s treatment could be assessed, using the other institution as a control group. Since the risk score generated by the tool will be used primarily by neonatologists and NICU nurses, it becomes critical to have them express how they would like such a tool to be presented. Neonatologists, responsible for the management of the overall health of these infants, including other possible surgical interventions and their discharge or transfer, might have The Retinopathy of Prematurity Risk Assessment Tool 24 different needs than NICU nurses, who are often on the front lines with family communication. Additional education of NICU staff about the condition could be helpful at this point. This might be something as simple as a lecture. ROP is only one of many conditions that the NICU staff needs to consider with these children and their families, so information needs to be presented in a concise, yet informative way. The project leader, as the main advocate, will then introduce and discuss the ROPSRAT CDSS, discussing what it has to offer and its technical limitations. The project team must be prepared for resistance during the first meeting, and listen to the stakeholder’s concerns. They must fully understand the reasons behind the resistance as they can reveal obstacles better dealt with at the beginning stage. It may be that NICU nurses have a very different perspective on family communication than ophthalmologists and neonatologists, and such a perspective must be given due consideration. As a facilitator, it is important that the project leader, who is the change manager, build the bridge of communication as open as possible by encouraging users and other stakeholders to fully participate and contribute from project inception through conferences, meetings, consultations, and other collaborative events for decision-making and project milestones review. After the first forum, the change team must continually involve and communicate with other stakeholders in the project’s design, development, implementation, deployment, and evaluation. Planning and Development. Since this is a single score representing risk, it will likely not involve major changes in workflow. However it must be introduced with sensitivity to the existing burdens of a busy staff. Workshops can be done to review priorities with the stakeholders, reach agreement regarding short term and long term action plans, discuss implementation methods, and examine accountabilities. One potential technique that could be The Retinopathy of Prematurity Risk Assessment Tool 25 developed for use in a workshop would be to present nursing staff with three different risk scores, one for a child at high risk, one for a child at moderate risk, and one for a child at low risk. The approach to families with children at different risk levels should be different. Obviously, the parents of children at high risk need to understand the severity of the problem, but it is just important that the parents of children at low risk not be burdened with undue worry about a problem unlikely to affect their infant. By incorporating these stakeholders into the change process at this point, accepting change in their workflow will not be as difficult. There will be excitement rather than resistance as they know what and how change is coming. At this point, it will be possible to identify nurses and neonatologists to serve as clinical champions to help those less involved in the process understand how to use the risk score clinically. Once the CDSS is developed, the system must be tested prior to implementation, to determine where improvements or changes need to be made. Among other methods of evaluation, a successful user acceptance test will be critical to the successful deployment and adoption of the CDSS. Any errors or failures of the CDSS must be identified during the testing period, triaged and fully documented, and eventually remediated. Otherwise, end-user adoption may not follow which will invariably result in an overall project failure. Implementation. One advantage of involving stakeholders and end-users in the creation of the CDSS is providing them with a sense of ownership. Clinical champions and super users will be critical at this phase, who will serve as ambassadors of the new CDSS. They should be available and ready to assist end-users when needed, particularly at the time when the CDSS will be utilized the most. Staff surveys initially may not be helpful but after implementation could provide crucial feedback about issues and needs for improvement. During the first few weeks after rollout, the project team members must be available to answer questions and help The Retinopathy of Prematurity Risk Assessment Tool 26 interpretation of the results. During the initial deployment stage, allowing end-users and other stakeholders to provide input, through face-to-face meetings or surveys, regarding needed changes or improvements will also expedite the successful adoption of the CDSS. Post Implementation. A few weeks after initial deployment, it is important to communicate success indicators by reporting the results of measurable objectives. Initial accomplishments and continuing achievements must be noted, acknowledged, and celebrated. Part of the ongoing and continuous improvement of this change is to give individual and group credits to fortify the change in the organization. Another part of this post-implementation period is to evaluate successes and failures to allow for self-reflection on where individuals can improve for another possible project (“Change management, n.d.). Clearly, ROP is only one of many threats that a premature infant faces. A similar approach could be taken for a number of other common conditions. Evaluation Evaluation of the ROPSRAT will be an ongoing process that begins from the early stage of development and continuously during its implementation. Usability of the ROPSRAT CDSS is integral to its adoption by clinicians and the achievement of its goals and objectives. As recommended by Horsky et al. (2010) response to clinician emails, online surveys, observations, and interviews will play a great role in evaluating the ROPSRAT design at different stages. The assessment will cover the following measures as suggested by Osheroff et al. (2012): system response time, structure, process, and outcome. The Retinopathy of Prematurity Risk Assessment Tool 27 System Response Time As proposed, system response time of the ROPSRAT CDSS should have the least amount of response time as possible to promote user acceptance. If the EMR does not deliver the recommendations in a timely way, the ophthalmologists may become frustrated and totally disregard the recommendations. Therefore, the ROPSRAT should be evaluated based on rapid response and availability at the point of care when the physicians enter their notes and whether it adversely affects the efficiency of physician’s workflow; otherwise, the ROPSRAT recommendations may not be well received by system users. Structure It is vital to measure how the ROPSRAT CDSS will be presented and incorporated in the workflow of the users. The structure will be measured in terms of the nature and number of alerts. The evaluation of the alerts will be based on whether they are configured and triggered appropriately in critical stages of the workflow and whether the buttons are friendly and easy to use. The order set will also be evaluated whether it is relevant and deployed at the right time. To be specific, the order set needs to come right after or alongside the recommendation alert (see Figure 3) for the next eye examination schedule. This way, the ophthalmologist will look at the recommendation first then move on to ordering the next eye examination. It is also imperative to evaluate that the buttons and the presentation of the tool so as to ensure that the order sets are relevant and simple to use. Process The clinical recommendations table derived from the recent recommendations for the management of retinopathy of prematurity will be reviewed by the ophthalmologists and the neonatologists on the project team. Although these guidelines are intended to be straightforward, The Retinopathy of Prematurity Risk Assessment Tool 28 there is some room for interpretation. It is important that uncertainty and disagreements are acknowledged. It may be that in certain circumstances there are no clear recommendations. The decision-support tool must clearly communicate this uncertainty to the user. Perhaps more challenging will be validation of the implementation. Rules for follow up and treatment must be entered into the Epic system in order to provide appropriate responses. Perhaps the best way to validate this is to have the lead clinician on the project team use the tool for a period of time prior to releasing it to other users. Any discrepancies or concerns must be noted for review later. An effective way to do this may be by email to a central development location. If the ophthalmologist does not have the immediate ability to comment on any potential discrepancies, it may be difficult to remember them later. Validation of the quantitative estimate of risk may be more problematic. When data had been collected on only a small number of patients, it is possible and perhaps likely that a few outliers might throw off the risk calculation dramatically. For example, if a set of triplets with the same birth age and similar birth weights all go on to need laser surgery, other infants with similar statistics might have an artificially elevated calculated risk. By periodically performing the multivariate regression analysis and updating coefficients that will be used to calculate the risk for each infant, as the data pool increases, the estimates will become increasingly accurate. It must be stressed that users of the system should be made aware of this difficulty, especially in the first few years after deployment. Moreover, the ROPSRAT process should be evaluated in terms of how often the intervention is used, how the alert is utilized, how users respond to the alert, the adoption rate of the users, the characteristics of the users in relation to adoption rates, effects of the intervention in the workflow, and whether the users find the intervention helpful, satisfying, or distracting. The Retinopathy of Prematurity Risk Assessment Tool 29 Human-technology interface issues should also be identified if encountered as this will affect workflow efficiency and user acceptance. Outcome Another metric that should be measured is the effect of ROPSRAT implementation in the decreased incidence of blindness and critical ROP or decreased length of stay among premature infants. With ROPSRAT providing follow-up recommendations in a timely manner, there will be more consistency and accuracy in terms of follow-up eye exam recommendations and treatment. This in turn should create a positive visual health outcome among premature infants affected by ROP. The rate of consistency among ophthalmologists in terms of following clinical guidelines should be measured as stated in the objective. Increased family satisfaction from improved communication of ROP status should be measured as well. The risk management score will not be helpful in communicating with families if it is ignored. Its use will depend on how nurses perceive their relevance and clinical value. It is important to evaluate the nursing staff’s opinion of the tool and to take corrective actions to address common issues and concerns through adequate training and education. Employing surveys to help nurses improve their interactions with families would be ideal. Parental satisfaction with the process may be difficult to quantify. However, surveys or structured interviews may be done before deployment to establish a baseline. Families may be satisfied with their babies ROP treatment due to lack of education about their child’s risk. Connecticut Children’s dual NICU structure may allow one institution to serve as a control group in assessing satisfaction after the introduction. The Retinopathy of Prematurity Risk Assessment Tool 30 Adoption rate among users should also be evaluated. Since decision making for treatment and follow-up recommendations is repeated for each evaluation, the ROPSRAT tool will help physicians decrease the time spent on decision-making. It is important to evaluate whether the system provides alerts to intended users at the time of decision making process; whether it is performing its role as an assistive tool rather than an irritation. Discussion The portion of ROPSRAT that provides recommendations for treatment or follow-up can be implemented as soon as it is sufficiently evaluated. Clearly it will take longer for the probability estimate to be ready. Several years of data will be needed before quantitative estimates can be provided, although hopefully qualitative estimates of “low,” “moderate,” and “high” risk can be generated within six months to a year. It is essential that users of the system understand its limitations. If not, it is possible that after a few experiences with clearly meaningless scores, the NICU staff may develop the habit of ignoring them, even as they become more meaningful. The limitation of the risk assessment model, at least as it stands now, is that it has not incorporated all potential risk factors into the calculation of the risk score. A multivariate regression analysis will show how much of the variability has been explained with the factors that have been taken into account. If only a relatively small amount of variability is incorporated, say 40%, then the remaining 60% of the variability has not been incorporated into the model. Data mining techniques can assist in trying to determine what other risk factors should be incorporated. Our society is in the process of transitioning to a new model of healthcare financing. Insurers are very interested in improving quality outcomes and this tool could provide a dramatic The Retinopathy of Prematurity Risk Assessment Tool 31 demonstration of efforts to improve quality. Additionally, ophthalmologists, and the hospitals that employ them, are paid by insurers for every exam they do on premature infants. There is currently no incentive to limit the number of exams performed, other than that the exams themselves can be traumatic for the infant. As we move towards more accountable care, the model and the data it generates could be used to perhaps modify the clinical guidelines. If for example infants born between 1100 and 1200 g at 28 weeks have never gone on to develop the need for surgery after several years of data accumulation, and the calculated risk is zero, perhaps fewer exams could be done. This of course must be a step taken with caution since a mistake could result in blindness, but the approach should be considered to reduce unnecessary examinations. The Retinopathy of Prematurity Risk Assessment Tool 32 References American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus & American Association of Certified Orthoptists, 2013. Screening examination for premature infants for retinopathy of prematurity. Pediatrics 131:189-195. patient satisfaction. Day, S., Menke, A.M., & Abbott, R.L. (2009). Retinopathy of Prematurity malpractice claims. Archives of Ophthalmology 127(6):794-798. Early Treatment for Retinopathy of Prematurity Cooperative Group, 2003. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Archives of Ophthalmology. 121:1684 – 1696 Facts about retinopathy of prematurity (rop). (n.d.). Retrieved from http://www.nei.nih.gov/health/rop/rop.asp Hardin, J.M., Chhieng, D.C. (2007). Data mining and clinical decision support systems. In: Clinical decision support systems: Theory and practice, Berner, E.S., ed., New York, NY, USA: Springer, Health Informatics Series. Hunink, M., Glasziou, P., Siegel, J., Weeks, J., Pliskin, J., Elstein, A., & Weinstein, M. (2001). Decision-making in health and medicine: Integrating evidence and values. Cambridge, UK: Cambridge University Press ICD-10 Data.com, 2014. Retrieved from http://www.icd10data.com/ICD10CM/Codes/H00H59/H30-H36/H35-. The Retinopathy of Prematurity Risk Assessment Tool 33 International Committee for the Classification of Retinopathy of Prematurity, 2005. The international classification of retinopathy of prematurity revisited. Archives of Ophthalmology 123:991-999. Osheroff, J., Teich, J., Levick, D., Saldana, L., Velasco, F., Sittig, D., Rogers, K., & Jenders, R. (2012).Improving outcomes with clinical decision support. An implementer's guide. (2nd ed.). Stanton, M. (2006, June). The high concentration of U.S. health care expenditures. Retrieved from http://www.ahrq.gov/research/findings/factsheets/costs/expriach/index.html WHO. (2007). Vision 2020 the right to sight. Retrieved from http://www.who.int/blindness/Vision2020_report.pdf The Retinopathy of Prematurity Risk Assessment Tool 34 Table Decision Logic ZONE 1 1 1 1 1 1 1 1 1 1 1 1 2 2 2 2 2 2 2 2 2 2 2 2 3 3 STAGE 0 0 0 1 1 1 2 2 2 3 3 3 0 0 0 1 1 1 2 2 2 3 3 3 0 0 VESSELS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS PLUS NO PLUS PRE-PLUS RESPONSE FU 1 WEEK FU 1 WEEK TREATMENT FU 1 WEEK FU 1 WEEK TREATMENT FU 1 WEEK FU 1 WEEK TREATMENT TREATMENT TREATMENT TREATMENT FU 2 WEEKS FU 1 WEEK TREATMENT FU 2 WEEKS FU 1 WEEK TREATMENT FU 1 WEEK FU 1 WEEK TREATMENT FU 1 WEEK FU 1 WEEK TREATMENT FU 2 WEEKS FU 1 WEEK 3 0 PLUS TREATMENT NOTES CLINICALLY UNLIKELY CLINICALLY UNLIKELY 3 1 NO PLUS FU 2 WEEKS 3 1 PRE-PLUS FU 1 WEEK 3 1 PLUS TREATMENT 3 2 NO PLUS FU 1 WEEK 3 2 PRE-PLUS FU 1 WEEK 3 2 PLUS TREATMENT 3 3 NO PLUS FU 1 WEEK 3 3 PRE-PLUS FU 1 WEEK 3 3 PLUS TREATMENT RETINA MATURE FU 6-9 MONTHS ROP REGRESSED FU 6-9 MONTHS Adapted from American Academy of Pediatrics Section on Ophthalmology et al. (2013). The Retinopathy of Prematurity Risk Assessment Tool 35 Figures Figure 1 Goals and Objectives Organizational Goals: Improve the visual health among premature infants, and optimize cost-effectiveness of visual care. Clinical Goals: 1. Improve the quality of ROP care and enhance communcation among ophthalmologist, NICU staff, and families. Develop an accurate recommendation tool for followup and treatment of ROP that is timely integrated in clinician's workflow utilizing the most current guidelines set by AAP. Develop a tool to quantify the risk of ROP progression for utilization by ophthalmologist and NICU staff. Develop a tool that allows quantitative risk comparison across NICUs . Develop a tool that allows quantitative comparison of ophthalmologist’s performance or practice . The Retinopathy of Prematurity Risk Assessment Tool Figure 2 Pop-up screen showing recommended follow up. 36 The Retinopathy of Prematurity Risk Assessment Tool 37 Figure 3 Sample letter to families of infants with stage 2/zone 2/no plus ROP and 25% calculated risk of progression to needing laser surgery. To the parents of <INFANT NAME>: <INFANT NAME>’s eyes were examined today for a condition called retinopathy of prematurity. This is a disease that affects the eyes of premature infants and can cause complete and permanent blindness. Fortunately, a good treatment is available for this condition that can prevent blindness in most cases. But it is critical that the disease is identified in a timely way. Your baby has a <MODERATE> risk of progression to needing laser surgery. The eye is a very complex organ. It involves multiple parts that need to work together to achieve optimal vision. The tissue inside the eye that senses light is called the retina. It requires a good blood vessel supply in order to function. When your premature infant was born, <HIS (HER)> retinal blood vessels were not fully complete. The premature birth has caused the blood vessels to develop inappropriately. Instead of growing along the retina, they are growing into the center of the eye. If this becomes severe enough, the blood vessels can tear the retina off the back of the eye causing a complete and permanent retinal detachment and complete blindness. Right now, your baby has a form of the disease that is not severe enough to cause blindness, but it could progress to that point. Based on our experience at Connecticut Children’s Medical Center, we estimate that your baby has a <25%> chance of progression to that point. <HIS (HER)> doctors and nurses are aware of this risk and are taking precautions to make certain that this risk is minimized. However, it is critical that <HE (SHE)> be examined again in one week. Dr. <OPHTHALMOLOGIST> will round next week and will provide you with an update after that exam. If <INFANT NAME> is discharged prior to next week’s scheduled rounds, it is critical that you keep the follow-up visit arranged in the outpatient retinopathy of prematurity follow-up clinic. Missing even one visit could result in permanent blindness. Your ophthalmologist, Dr. <OPHTHALMOLOGIST>, is available if you would have additional questions. Please call at 860 – XXX – XXXX should you wish to discuss this further. The Retinopathy of Prematurity Risk Assessment Tool 38 Figure 4 Stakeholder Workflow for the Follow-up Recommendation Tool and Order set NICU Nurse Inputs patient data in EHR Neonatologist Receives alert for eye examination recommendation. Or Receives risk score calculation Ophthalmologist Receives list of patients to be seen for eye examination Examines patient Receives and carries out orders for eye exam schedule Receives order on scheduled eye examination from ophthalmol ogist and fluid and nutrition order from neonatologi st Order Set (for first eye examination and fluid and nutrition orders) Enters medical notes Receives alert on eye examination schedule and/or treatment recommendation Receives alert on scheduled eye examination made by ophthalmologist Order Set (for next eye examination) Order Set for fluid and nutrition