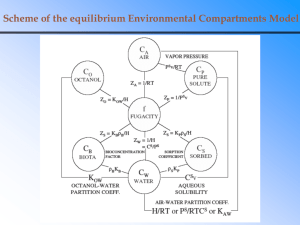
Homogeneous vs. heterogeneous: see Table 9.1.
advertisement

Chemistry 30A Dr Schaleger Chapter 9 Notes Solutions Laney College Spring 2014 Suggested homework: 9: 49,51,59,61,65,69,77,81,87,99,105,107. Homogeneous vs. heterogeneous: see Table 9.1. Gases are mutually soluble with one another, e.g., air. Liquid-liquid, gas in liquid, solid in liquid are most common solution types Factors that affect solubility. Dipole-dipole attraction; hydrogen bonding; London dispersion forces. Concentrations units: mass of solute per mass of solution (%m/v). volume of solute per volume of solution, (%v/v). Most important in chemistry: molarity (M), #moles per liter of solution (typically aqueous). Dilutions: M1*V1 = M2*V2. Example: 10 mL of a 0.25 M solution of NaOH is diluted to a final volume of 250 mL. What is the final concentration? Answer: M2 = 0.25*10/250 = 0.010 M. (N0te that units are consistent, that is, mL and mL. That is, no need to convert to liters as long as units are consistent. Electrolyte: definition. An electrolyte is a solution that conducts an electric current. Any ionic compound that is solutble in water functions as an electrolyte. Weak electrolyte: Typically these are weak molecular acids and weak molecular bases like acetic acid and ammonia that are partially dissociated or ionized in aqueous solution (wait for Chapter 10 for a more complete discussion), Colligative properties: depend on concentration of the solution or mixture. Vapor pressure lowering (boiling point elevation) Freezing (melting) point depression. Osmosis: driving force is due to the tendency to achieve equilibrium, that is, two unequal concentrations tend to equilibrate at a common concentration. General rule: “like dissolves like”. Polar solvents for ionic compounds and polar molecules: water, sulfuric acid, methanol, acetone. Nonpolar solvents for nonpolar molecules: liquid hydrocarbons, carbon tetrachloride. Fats and oils consist of a long, nonpolar hydrocarbon tail and a polar head. Soap is the sodium salt of a long-chain, nonpolar fatty acid. How does soap work as a cleaning agent? Miscibility: infinitely soluble, mutuallysoluble in all proportions like ethanol and water, hexane and octane. 1 Chemistry 30A Dr Schaleger Chapter 9 Notes Solutions Laney College Spring 2014 Effect of temperature on solubility: solubility is an equilibrium phenomenon so think thermodynamics: M(s) = M(aq) and ΔG = ΔH – TΔS. Typically the heat of solution is positive (why?) and the entropy of solution is positive (why?), so higher temperatures tend to increase solubility. (Not always true however). Henry’s Law. Applies to solutions of gases in liquid. The concentration of the gas in the liquid is directly proportional to vapor pressure. Consider the carbon dioxide under pressure in a soda pop can: CO2(aq) = CO2(g). The Henry’s law expression is [CO2] = kpCO2. As the can is opened, the pressure of CO2 in the vapor phase is decreased so in order to maintain equilibrium (k is a constant), dissolved CO2 comes out of solution: the solution ‘fizzes’. (Recall LeChatelier’s principle: if the equilibrium is disturbed, the system reacts in a way so as to restore the equilibrium). Example in text: Solubility of oxygen in blood at an elevation of 11,000 ft. (Solubility decreases as a result of decreased concentration of oxygen in the air at that altitude: Henry’s law in action!). Note that as in other examples of equilibtia, [C1]/[C2] = p1/p2. 2