BSP 170: MATERIALS SCIENCE Imperfections in the Atomic and
advertisement

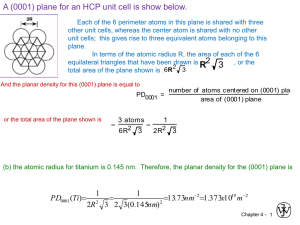
BSP 170: MATERIALS SCIENCE Imperfections in the Atomic and Ionic Arrangements 2013 Objectives Introduce the three basic types of imperfections: point defects, line defects (or dislocations), and surface defects. Explore the nature and effects of different types of defects. Outline Point Defects Dislocations Observing Dislocations Significance of Dislocations Influence of Crystal Structure Surface Defects Importance of Defects Point defects - Imperfections, such as vacancies, that are located typically at one (in some cases a few) sites in the crystal. Extended defects - Defects that involve several atoms/ions and thus occur over a finite volume of the crystalline material (e.g., dislocations, stacking faults, etc.). Vacancy - An atom or an ion missing from its regular crystallographic site. Interstitial defect - A point defect produced when an atom is placed into the crystal at a site that is normally not a lattice point. Substitutional defect - A point defect produced when an atom is removed from a regular lattice point and replaced with a different atom, usually of a different size. Frenkel defect - A vacancy-interstitial pair formed when an ion jumps from a normal lattice point to an interstitial site, as in Figure 4-1(e) leaving behind a vacancy. Schottky defect - A cation vacancy – anion vacancy pair created by removing one cation and one anion from the interior of the crystal and then placing them both at an external surface. Point Defects At room temperature (298 K), the concentration of vacancies is small, but the concentration of vacancies increases exponentially as the temperature increases, as shown by the following Arrhenius type behavior: −𝑄𝑣 (1) 𝑁𝑣 = 𝑁exp ( ) 𝑅𝑇 where 1 𝑁𝑣 is the number of vacancies per cm3 ; N is the number of atoms per cm3 ; 𝑄𝑣 is the energy required to produce one mole of vacancies, in cal mol or Joules mol; 𝑅 is the gas constant, or ; and 𝑇 is the temperature in degrees Kelvin. Figure 1. Point defects: (a) vacancy, (b) interstitial atom, (c) small substitutional atom, (d) large substitutional atom, (e) Frenkel defect, (f) Schottky defect. All of these defects disrupt the perfect arrangement of the surrounding atoms. Example 1. Vacancy Concentrations in Iron Determine the number of vacancies needed for a BCC iron crystal to have a density of 7.87 g/cm3 . The lattice parameter of the iron is 2.866 × 10−8 cm. Solution: The expected theoretical density of iron can be calculated from the lattice parameter and the atomic mass. (2 atoms/cell)(55.847 g/mol) 𝜌= = 7.8814 g/cm3 (2.866 × 10−8 cm)3 (6.02 × 1023 atoms/mol) Let’s calculate the number of iron atoms and vacancies that would be present in each unit cell for the required density of 7.87 g/cm3: 𝜌= (𝑛 atoms/cell)(55.847 g/mol) = 7.87 g/cm3 (2.866 × 10−8 cm)3 (6.02 × 1023 atoms/mol) 𝑛 atoms/cell = (7.87)(2.866 × 10−8 )3 (6.02 × 1023 ) = 1.9971 (55.847) 2 Or, there should be 2.00 – 1.9971 = 0.0029 vacancies per unit cell. The number of vacancies per cm3 is: 0.0029 vacancies/cell Vacancies⁄cm3 = = 1.23 × 1020 (2.866 × 10−8 cm)3 Note that other defects such as grain boundaries in a polycrystalline material contribute to a density lower than the theoretical value. Example 2 The Effect of Temperature on Vacancy Concentrations Calculate the concentration of vacancies in copper at room temperature (25°𝐶). What temperature will be needed to heat treat copper such that the concentration of vacancies produced will be 1000 times more than the equilibrium concentration of vacancies at room temperature? Assume that 20,000 cal are required to produce a mole of vacancies in copper. Solution: The lattice parameter of FCC copper is 0.36151 nm. There are four atoms per unit cell; therefore, the number of copper atoms per cm3 is 𝑁= 4 atoms/cell = 8.466 × 1022 copper atoms/cm3 (3.6151 × 10−8 cm)3 At room temperature, 𝑇 = 25 + 273 = 298 K: cal −𝑄𝑣 𝑁𝑣 = 𝑁exp ( 𝑅𝑇 ) = (8.466 × −20,000 atoms 1022 cm3 ) exp [ 1.987cal mol ] ( )(298K) = 1.814 × 108 mol.K vacancies cm3 We wish to find a heat treatment temperature that will lead to a concentration of vacancies that is 1000 times higher than this number, or 𝑁𝑣 = 1.814 × 1011 vacancies/ cm3 . We could do this by heating the copper to a temperature at which this number of vacancies forms: −𝑄𝑣 −20,000 𝑁𝑣 = 1.814 × 108 = 𝑁exp ( ) = (8.466 × 1022 )exp [ ] (1.987𝑇) 𝑅𝑇 exp [ −20,000 1.814 × 108 ]= = 0.214 × 10−11 (1.987𝑇) 8.466 × 1022 −20,000 = ln(0.214 × 10−11 ) = −26.87 1.987𝑇 𝑇= 20,000 = 375 K = 102℃ (1.987)(26.87) 3 By heating the copper slightly above 100°𝐶, waiting until equilibrium is reached, and then rapidly cooling the copper back to room temperature, the number of vacancies trapped in the structure may be one thousand times greater than the equilibrium number of vacancies at room temperature. Thus, vacancy concentrations encountered in materials are often dictated by both thermodynamic and kinetic factors. Interstitial Defects Figure 2 The location of the ¼, ½, 0 interstitial site in BCC metals, showing the arrangement of the normal atoms and the interstitial atom (b) ½, 0, 0 site in FCC metals. (c) Edge centers and cube centers are some of the interstitial sites in the FCC structure. An interstitial defect is formed when an extra atom or ion is inserted into the crystal structure at a normally unoccupied position, as in Figure 1 (b). Interstitial atoms or ions, although much smaller than the atoms or ions located at the lattice points, are still larger than the interstitial sites that they occupy; consequently, the surrounding crystal region is compressed and distorted. Interstitial atoms such as hydrogen are often present as impurities, whereas carbon atoms are intentionally added to iron to produce steel. For small concentrations, carbon atoms occupy interstitial sites in the iron crystal structure, introducing a stress in the localized region of the crystal in their vicinity. As we will see, the introduction of interstitial atoms is one important way of increasing the strength of metallic materials. Unlike vacancies, once introduced, the number of interstitial atoms or ions in the structure remains nearly constant, even when the temperature is changed. We could calculate the size of the interstitial site at the 1/4, 1/2, 0 location. The radius 𝑅BCC of the iron atom is: 4 𝑅𝐵𝐶𝐶 = We find that: √3𝑎 (√3)(0.2866) = = 0.1241 nm 4 4 1 2 1 2 ( 𝑎) + ( 𝑎) = (𝑟interstitial + 𝑅𝐵𝐶𝐶 )2 2 4 𝑟𝑖𝑛𝑡𝑒𝑟𝑠𝑡𝑖𝑡𝑖𝑎𝑙 = √0.02567 − 0.1241 = 0.0361 nm For FCC iron, the interstitial site such as the 1/2, 0, 0 lies along ⟨100⟩ directions. Thus, the radius of the iron atom and the radius of the interstitial site are: 𝑅𝐹𝐶𝐶 = √2𝑎 (√2)(0.3571) = = 0.1263 nm 4 4 2𝑟interstitial + 2𝑅𝐹𝐶𝐶 = 𝑎 𝑟interstitial = 03571 − 2(0.1263) = 0.0522 nm 2 The interstitial site in the BCC iron is smaller than the interstitial site in the FCC iron. Although both are smaller than the carbon atom, carbon distorts the BCC crystal structure more than the FCC crystal. As a result, fewer carbon atoms are expected to enter interstitial positions in BCC iron than in FCC iron. Frenkel and Schottky Defects The equilibrium numbers of both Frenkel and Schottky defects increase with and depend on temperature in a manner similar to the number of vacancies in metals (Equation 1). For Frenkel defects, the number of cation-vacancy/cation-interstitial defect pairs (𝑁𝑓𝑟 ) depends on temperature according to the following expression: 𝑄𝑓𝑟 𝑁𝑓𝑟 = 𝑁exp (− ) (2) 2𝑘𝑇 Here 𝑄𝑓𝑟 is the energy required for the formation of each Frenkel defect, and N is the total number of lattice sites. The factor 2 is present in the denominator of the exponential because two defects (a missing cation and an interstitial cation) are associated with each Frenkel defect. Similarly, for Schottky defects, in an AX-type compound, the equilibrium number (𝑁𝑠 ) is a function of temperature as 𝑄𝑠 𝑁𝑠 = 𝑁exp (− ) (3) 2𝑘𝑇 When 𝑄 is given in joules, 𝑘 = 1.38 × 10−23 J/atom-K. When using 𝑒𝑉 as the unit of energy, k = 8.62 × 10-5 eV/atom-K. When 𝑄 is given in joules/mol, then use 𝑅 (the universal or molar gas constant) instead of 𝑘. The value of 𝑅 is 8.314 𝐽𝐾 −1 mol−1 or 1.987 calK −1 mol−1 so 5 take care when using this Arrhenius type equation. Note carefully the units in which the activation energy is given and then use the appropriate constant. Is the activation energy given in joules per mol, calories per mol, joules per atom or electron-volt per atom? Consult the following table whenever necessary. Table 1 Units of Activation Energy and Appropriate Constant to Use Constant to be Used Units of Activation Energy 𝑸𝒗 eV/atom 𝑘 = 8.62 × 10−5eV/atom-K J/atom 𝑘 = 1.38 × 10−23J/atom-K J/mol 𝑅 = 8.314 JK −1 mol−1 cal/mol 𝑅 = 1.987 calK −1 mol−1 Example 3. Calculating the number of Schottky defects Calculate the number of Schottky defects per cubic metre in potassium chloride at 500℃. The energy required to form each Schottky defect is 2.6 eV, while the density of KCl is 1.955 g/cm3 . Solution: To solve this problem it is necessary to use Equation 3. However, we must first compute the value of N (the number of lattice sites per cubic metre); this is possible using a modified form of the density equation. 𝑁= 𝑁𝐴 𝜌 𝐴𝐾 + 𝐴𝐶𝑙 where 𝑁𝐴 is the Avogadro number, 𝜌 is the density, and 𝐴𝐾 and 𝐴𝐶𝑙 are the atomic weights for potassium and chlorine respectively. Therefore, 𝑁= (6.022 × 1023 atoms/mol)(1.955 g/cm3 )(106 cm3 /m3 ) = 1.58 × 1028 sites/m3 39.10 g/mol + 35.45 g/mol From equation 3, 𝑁𝑠 = 𝑁 × 𝑒𝑥𝑝 (− 𝑄𝑠 ) 2𝑘𝑇 = (1.58 × 1028 sites/m3 )exp [− 2.6 eV (2)(8.62 × 10−5 eV/K)(500 = 5.31 × 1019 defects /m3 . Dislocations 1. Dislocation - A line imperfection in a crystalline material. 6 + 273 K) ] 2. 3. 4. 5. Screw dislocation - A dislocation produced by skewing a crystal so that one atomic plane produces a spiral ramp about the dislocation. Edge dislocation - A dislocation introduced into the crystal by adding an ‘‘extra half plane’’ of atoms. Mixed dislocation - A dislocation that contains partly edge components and partly screw components. Slip - Deformation of a metallic material by the movement of dislocations through the crystal. Dislocations are line imperfections in an otherwise perfect crystal. They typically are introduced into a crystal during solidification of the material or when the material is deformed permanently. Although dislocations are present in all materials, including ceramics and polymers, they are particularly useful in explaining deformation and strengthening in metallic materials. We can identify three types of dislocations: the screw dislocation, the edge dislocation, and the mixed dislocation. Figure 3 Perfect crystal (a) is cut and sheared one atom spacing, (b) and (c). The line along which shearing occurs is a screw dislocation. A Burgers vector b is required to close a loop of equal atom spacings around the screw dislocation. Figure 4 The perfect crystal in (a) is cut and an extra plane of atoms is inserted (b). The bottom edge of the extra plane is an edge dislocation (c). A Burgers vector b is required to close a loop of equal atom spacings around the edge dislocation. 7 Dislocation Motion Consider the edge dislocation shown in Figure 5(a). A plane that contains both the dislocation line and the Burgers vector is known as a slip plane. When a sufficiently large shear stress acting parallel to the Burgers vector is applied to a crystal containing a dislocation, the dislocation can move through a process known as slip. The bonds across the slip plane between the atoms in the column to the right of the dislocation shown are broken. The atoms in the column to the right of the dislocation below the slip plane are shifted slightly so that they establish bonds with the atoms of the edge dislocation. In this way, the dislocation has shifted to the right [Figure 5(b)]. If this process continues, the dislocation moves through the crystal [Figure 5(c)] until it produces a step on the exterior of the crystal [Figure 5(d)] in the slip direction (which is parallel to the Burgers vector). (Note that the combination of a slip plane and a slip direction comprises a slip system.) The top half of the crystal has been displaced by one Burgers vector relative to the bottom half; the crystal has been plastically (or permanently) deformed. This is the fundamental process that occurs many, many times as you bend a paper clip with your fingers. The plastic deformation of metals is primarily the result of the propagation of dislocations. This process of progressively breaking and reforming bonds requires far less energy than the energy that would be required to instantaneously break all of the bonds across the slip plane. The crystal deforms via the propagation of dislocations because it is an energetically favorable process. Figure 5 (a) When a shear stress is applied to the dislocation in (a), the atoms are displaced, (b) causing the dislocation to move one Burgers vector in the slip direction. (c) Continued movement of the dislocation eventually creates a step (d), and the crystal is deformed. 8 A caterpillar only lifts some of its legs at any given time rather than lifting all of its legs at one time in order to move forward. Why? Because lifting only some of its legs requires less energy; it is easier for the caterpillar to do. Another way to visualize this is to think about how you might move a large carpet that is positioned incorrectly in a room. If you want to reposition the carpet, instead of picking it up off the floor and moving it all at once, you might form a kink in the carpet and push the kink in the direction in which you want to move the carpet. The width of the kink is analogous to the Burgers vector. Again, you would move the carpet in this way because it requires less energy—it is easier to do. Slip Figure 6(a) is a schematic diagram of an edge dislocation that is subjected to a shear stress 𝝉 that acts parallel to the Burgers vector and perpendicular to the dislocation line. In this drawing, the edge dislocation is propagating in a direction opposite to the direction of propagation shown in Figure 5(a). For an edge dislocation, a component of the shear stress must act parallel to the Burgers vector (and perpendicular to the dislocation line) in order for the dislocation to move. The dislocation line moves in a direction parallel to the Burgers vector. Figure 5(b) shows a screw dislocation. For a screw dislocation, a component of the shear stress must act parallel to the Burgers vector (and thus the dislocation line) in order for the dislocation to move. The dislocation moves in a direction perpendicular to the Burgers vector, and the slip step that is produced is parallel to the Burgers vector. Since the Burgers vector of a screw dislocation is parallel to the dislocation line, specification of the Burgers vector and dislocation line does not define a slip plane for a screw dislocation. Figure 6 Schematic of slip line, slip plane, and slip (Burgers) vector for (a) an edge dislocation and (b) for a screw dislocation. Burger’s Vector The Burgers vector is a precise statement of the magnitude and direction of shear that a dislocation produces. It is defined by means of a circuit around the dislocation on any surface which intersects the dislocation. 9 Figure 7 Illustration of Burgers circuit and Burgers vector for an edge dislocation: (a) a Burgers circuit closes upon itself when it surrounds a dislocation- free region of a crystal: (b) when the Burgers circuit surrounds a dislocation, the start and stop points are not coincident and the vector pointing from the stop point to the start point is defined to be the Burger’s vector for the dislocation. The vector's magnitude and direction is best understood when the dislocation-bearing crystal structure is first visualized without the dislocation, that is, the perfect crystal structure. In this perfect crystal structure, a rectangle whose lengths and widths are integer multiples of "a" (the unit cell length) is drawn encompassing the site of the original dislocation's origin. Once this encompassing rectangle is drawn, the dislocation can be introduced. This dislocation will have the effect of deforming, not only the perfect crystal structure, but the rectangle as well. The said rectangle could have one of its sides disjoined from the perpendicular side, severing the connection of the length and width line segments of the rectangle at one of the rectangle's corners, and displacing each line segment from each other. What was once a rectangle before the dislocation was introduced is now an open geometric figure, whose opening defines the direction and magnitude of the Burgers vector. Specifically, the breadth of the opening defines the magnitude of the Burgers vector, and, when a set of fixed coordinates is introduced, an angle between the termini of the dislocated rectangle's length line segment and width line segment may be specified. The direction of the vector depends on the plane of dislocation, which is usually on the closestpacked plane of unit cell. The magnitude is usually represented by the equation: ‖𝐛‖ = 𝑎 √ℎ 2 + 𝑘 2 + 𝑙 2 2 (4) where 𝑎 is the unit cell length of the crystal, ||b|| is the magnitude of Burgers vector and ℎ, 𝑘, and 𝑙 are the components of the Burgers vector, b = <h k l>. In most metallic materials, the magnitude of the Burgers vector for a dislocation is of a magnitude equal to the interatomic spacing of the material, since a single dislocation will offset the crystal lattice by one closepacked crystallographic spacing unit. 10 In edge dislocations, the Burgers vector and dislocation line are at right angles to one another. In screw dislocations, they are parallel. The Burgers vector is significant in determining the yield strength of a material by affecting solute hardening, precipitation hardening and work hardening. Example 4. Dislocations in Ceramic Materials A sketch of a dislocation in magnesium oxide (MgO), which has the sodium chloride crystal structure and a lattice parameter of 0.396 nm, is shown in Figure 8. Determine the length of the Burgers vector. Figure 8 An edge dislocation in MgO showing the slip direction and Burgers vector Solution: In Figure 8, we begin a clockwise loop around the dislocation at point 𝒙 and then move equal atom spacings in the two horizontal directions and equal atom spacings in the two vertical directions to finish at point 𝒚. Note that it is necessary that the lengths of the two horizontal segments of the loop be equal and the lengths of the vertical segments be equal, but it is not necessary that the horizontal and vertical segments be equal in length to each other. The chosen loop must close in a perfect crystal. The vector b is the Burgers vector. Because b is parallel to a [110] direction, it must be perpendicular to (110) planes. The length of b is the distance between two adjacent (110) planes. From the equation for the distance between two adjacent planes, we have 𝑑110 = 𝑎 √ℎ2 + 𝑘 2 + 𝑙 2 = 0.396 √12 + 12 + 02 = 0.396 √2 = 0.396√2 0.280 nm 2 The Burgers vector is a (110) direction that is 0.280 nm in length. Note, however, that two extra half planes of atoms make up the dislocation—one composed of oxygen ions and one of magnesium ions (Figure 8). This formula for calculating the magnitude of the Burgers vector will not work for non-cubic systems. It is better to consider the magnitude of the Burgers vector as equal to the repeat distance in the slip direction. 11 Example 5 Burgers Vector Calculation Calculate the length of the Burgers vector in copper. Solution: Copper has an FCC crystal structure. The lattice parameter of copper (Cu) is 0.36151 nm. The close-packed directions, or the directions of the Burgers vector, are of the form ⟨110 ⟩. The repeat distance along the 110 directions is one-half the face diagonal, since lattice points are located at corners and centers of faces [Figure 9(a)]. Face Diagonal = √2a = (√2)(0.36151) = 0.51125 nm The length of the Burgers vector, or the repeat distance, is 𝒃 = Figure 9 1 1 (Face Diagonal) = (0.51125) = 0.25563 nm 2 2 (a) Burgers vector for FCC copper. (b) The atom locations on a (110) plane in a BCC unit cell (for Examples 5 and 6, respectively). Example 6 Identification of Preferred Slip Planes The planar density of the (112) plane in BCC iron is 9.94 × 1014 atoms/cm2 . Calculate (a) the planar density of the (110) plane and (b) the interplanar spacings for both the (112) and (110) planes. On which plane would slip normally occur? Solution: The lattice parameter of BCC iron is 0.2866 nm or 2.866 × 10−8 cm. The (110) plane is shown in Figure 9(b), with the portion of the atoms lying within the unit cell being shaded. Note that one-fourth of the four corner atoms plus the center atom lie within an area of 𝑎 times √2𝑎. . (a) The planar density is 12 Planar density (110) = atoms area =( 2 √2)(2.866×10−8 cm)2 Planar density (112) = 0.994 × 1015 atoms cm2 = 1.72 × 1015 atoms/cm2 (from problem statement) (b) The interplanar spacings are 𝑑110 = 2.866 × 10−8 √12 + 12 + 02 𝑑112 = = 2.0266 × 10−8 cm 2.866 × 10−8 √12 + 12 + 22 = 1.17 × 10−8 cm The planar density is higher and the interplanar spacing is larger for the (110) plane than for the (112) plane; therefore, the (110) plane is the preferred slip plane. Surface Defects Grain Boundaries The microstructure of many engineered ceramic and metallic materials consists of many grains. A grain is a portion of the material within which the arrangement of the atoms is nearly identical; however, the orientation of the atom arrangement, or crystal structure, is different for each adjoining grain. Three grains are shown schematically in Figure 10(a); the arrangement of atoms in each grain is identical but the grains are oriented differently. A grain boundary, the surface that separates the individual grains, is a narrow zone in which the atoms are not properly spaced. That is to say, the atoms are so close together at some locations in the grain boundary that they cause a region of compression, and in other areas they are so far apart that they cause a region of tension. Figure 10(b) shows a micrograph of grains in a stainless steel sample. One method of controlling the properties of a material is by controlling the grain size. By reducing the grain size, we increase the number of grains and, hence, increase the amount of grain boundary area. Any dislocation moves only a short distance before encountering a grain boundary, and the strength of the metallic material is increased. The Hall-Petch equation relates the grain size to the yield strength, 𝜎𝑦 = 𝜎0 + 𝐾𝑑 −1/2 (5) where 𝜎𝑦 is the yield strength (the level of stress necessary to cause a certain amount of permanent deformation), 𝑑 is the average diameter of the grains, and 𝜎0 and 𝐾 are constants for the metal. Recall from Chapter 1 that the yield strength of a metallic material is the minimum 13 Figure 10 (a) The atoms near the boundaries of the three grains do not have an equilibrium spacing or arrangement. (b) Grains and grain boundaries in a stainless steel sample. level of stress that is needed to initiate plastic (permanent) deformation. Figure 11 shows this relationship in steel. The Hall-Petch equation is not valid for materials with unusually large or ultrafine grains. In the chapters that follow, we will describe how the grain size of metals and alloys can be controlled through solidification, alloying, and heat treatment. Figure 11. The effect of grain size on the yield strength of steel at room temperature. Example 7 Design of a Mild Steel The yield strength of mild steel with an average grain size of 0.05 mm is 137.9MPa. The yield stress of the same steel with a grain size of 0.007 mm is 275.8 MPa. What will be the average grain size of the same steel with a yield stress of 206.8 MPa? Assume the HallPetch equation is valid and that changes in the observed yield stress are due to changes in grain size. 14 Solution: Using the Hall-Petch equation 𝜎𝑦 = 𝜎0 + 𝐾𝑑 −1/2 Thus, for a grain size of 0.05 mm, the yield stress is 137.9 = 𝜎0 + 𝐾(0.05)−1/2 Thus, for a grain size of 0.007 mm, the yield stress is 275.8 = 𝜎0 + 𝐾(0.007)−1/2 Solving these two equations, 𝐾 = 18.44 MPa∙ mm1/2 , and 𝜎0 = 55.5 MPa. Now we have the Hall-Petch equation as 𝜎𝑦 = 55.5 + 18.44𝑑 −1/2 If we want a yield stress of 206.9 MPa, the grain size should be 0.0148 mm. ASTM Grain Size Number (n) One manner by which grain size is specified is the ASTM grain size number (ASTM is the American Society for Testing and Materials). The number of grains per square inch is determined from a photograph of the metal taken at a magnification of 100. The ASTM grain size number n is calculated as 𝑁 = 2𝑛−1 (6) where 𝑁 is the number of grains per square inch. A large ASTM number indicates many grains, or a fine grain size, and correlates with high strengths for metals. Example 8 Calculation of ASTM Grain Size Number Suppose we count 16 grains per square inch in a photomicrograph taken at a magnification of 250. What is the ASTM grain size number? Solution: Consider one square inch from the photomicrograph taken at a magnification of 250. At a magnification of 100, the one square inch region from the 250 magnification image would appear as 100 2 2 1 in ( ) = 0.16 in2 250 and we would see 16 grains = 100 grains/in2 0.16 in2 15 Substituting in Equation 6, 𝑁 = 100 grains⁄in2 = 2𝑛−1 log 100 = (𝑛 − 1)log2 2 = (𝑛 − 1)(0.3010) 𝑛 = 7.64 Importance of Defects Extended and point defects play a major role in influencing mechanical, electrical, optical, and magnetic properties of engineered materials. In this section, we recapitulate the importance of defects on properties of materials. We emphasize that the effect of dislocations is most important in metallic materials. Effect on Mechanical Properties via Control of the Slip Process Any imperfection in the crystal raises the internal energy at the location of the imperfection. The local energy is increased because, near the imperfection, the atoms either are squeezed too closely together (compression) or are forced too far apart (tension). Defects in materials, such as dislocations, point defects, and grain boundaries, serve as “stop signs” for dislocations. They provide resistance to dislocation motion, and any mechanism that impedes dislocation motion makes a metal stronger. Thus, we can control the strength of a metallic material by controlling the number and type of imperfections. The following three common strengthening mechanisms are based on the three categories of defects in crystals. 1. 2. 3. Strain Hardening Solid-Solution Strengthening Grain-Size Strengthening QUESTIONS 1. Gold has 5.82 × 108 vacancies cm3 at equilibrium at 300 K. What fraction of the atomic sites is vacant at 600 K? 2. Calculate the number of vacancies per cm3 expected in copper at 1080°𝐶 (just below the melting temperature). The energy for vacancy formation is 20,000 cal mol. 3. The fraction of lattice points occupied by vacancies in solid aluminum at 660°𝐶 is 10−3 . What is the energy required to create vacancies in aluminum? 4. The density of a sample of FCC palladium is 11.98 g/cm3 , and its lattice parameter is 3.8902 Å. Calculate 16 (a) the fraction of the lattice points that contain vacancies; and (b) the total number of vacancies in a cubic centimeter of Pd. 5. The density of a sample of HCP beryllium is 1.844 g/cm3 , and the lattice parameters are 𝑎 = 0.22858 nm and 𝑐 = 0.35842 nm. Calculate (a) the fraction of the lattice points that contain vacancies; and (b) the total number of vacancies in a cubic centimeter. 6. BCC lithium has a lattice parameter of 3.5089 × 10−8 cm and contains one vacancy per 200 unit cells. Calculate (a) the number of vacancies per cubic centimeter; and (b) the density of Li. 7. FCC lead has a lattice parameter of 0.4949 nm and contains one vacancy per 500 Pb atoms. Calculate (a) the density; and (b) the number of vacancies per gram of Pb. 8. Cu and Ni form a substitutional solid solution. This means that the crystal structure of a Cu-Ni alloy consists of Ni atoms substituting for Cu atoms in the regular atomic positions of the FCC structure. For a Cu-30% wt.% Ni alloy, what fraction of the atomic sites does Ni occupy? 9. ZnS has the zinc blende structure. If the density is 3.02 g/cm3 and the lattice parameter is 0.59583 nm, determine the number of Schottky defects (a) per unit cell; and (b) per cubic centimeter 10. Draw a Burgers circuit around the dislocation shown in Figure 12. Clearly indicate the Burgers vector that you find. What type of dislocation is this? In what direction will the dislocation move due to the applied shear stress 𝝉? Reference your answers to the coordinate axes shown. . Figure 12 17 11. What are the Miller indices of the slip directions: (a) on the (111) plane in an FCC unit cell? (b) on the (011) plane in a BCC unit cell? 12. The crystal shown in Figure 13 contains two dislocations A and B. If a shear stress is applied to the crystal as shown, what will happen to dislocations A and B? Figure 13 13. What is meant by the terms plastic and elastic deformation? 14. Why is the theoretical strength of metals much higher than that observed experimentally? 15. How many grams of aluminum, with a dislocation density of 1010 cm/cm3 , are required to give a total dislocation length that would stretch from Kitwe to Lusaka (289 km)? 16. A single crystal of an FCC metal is oriented so that the [001] direction is parallel to an applied stress of 5000 psi. Calculate the resolved shear stress acting on the (111) slip plane in the [1̅10],[01̅1], and [101̅] slip directions. Which slip system(s) will become active first? 17. Arrange the following metals in the expected order of increasing ductility: Cu, Ti, and Fe. 18. A copper-zinc alloy has the following properties Grain diameter (mm) 0.015 0.025 0.035 0.050 Strength (MPa) 170 158 151 145 Determine 18 (a) the constants in the Hall-Petch equation; and (b) the grain size required to obtain a strength of 200 MPa. 19. For an ASTM grain size number of 8, calculate the number of grains per square inch (a) at a magnification of 100 and (b) with no magnification. 20. Determine the ASTM grain size number if 20 grains square inch are observed at a magnification of 400. 21. What makes plain carbon steel harder than pure iron? 22. Why is jewelry made from gold or silver alloyed with copper? 23. Why do we prefer to use semiconductor crystals that contain as small a number of dislocations as possible? 24. In structural applications (e.g., steel for bridges and buildings or aluminum alloys for aircraft), why do we use alloys rather than pure metals? 25. Do dislocations control the strength of a silicate glass? Explain. 26. What is meant by the term strain hardening? 27. To which mechanism of strengthening is the Hall-Petch equation related? 28. Pure copper is strengthened by the addition of a small concentration of Be. To which mechanism of strengthening is this related to? 19