File
advertisement

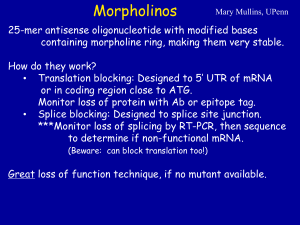
PSI- ©BIOL463 2014 Problem set 1 Answer the questions as completely and succinctly as you can, and provide clear justifications for your answers. You will have ample opportunity to discuss and review your answers in class, so give it your best effort, but please do not agonize over them. 1. Part I (at home; please hand in your answers to Q1, Part I in class!) The pictures below show the distributions of the bicoid (bcd) mRNA, the Bcd protein, the Caudal (Cad) protein, and the nanos (nos), orthodenticle (otd), oskar (osk) and hunchback (hb) mRNAs in early Drosophila embryos. bcd mRNA Bcd protein otd mRNA hb mRNA osk mRNA (red) Cad protein a) On your “embryo diagram” (PowerPoint slide for week 3), show the Bcd and Cad proteins, and nos and osk mRNAs. If you feel adventurous, also show the various zygotic mRNAs. b) What do the data show? The data show Bcd protein concentrated mostly on the outer region of the acron (in the anterior region), more diffuse at the outer regions of the future head, and very diffuse at the outer regions of the future thorax of the early Drosophila embryo. This contrasts with bcd mRNA, which is localized only at the tip of the anterior region. The data also shows a large distrubution for the Cad protein, which is highly concentrated at the posterior end and throughout the future abdomen but is not expressed in the anterior region. This protein diffuses as it enters the future thorax region of the developing embryo. Early Drosophila embryos appear to have osk mRNA localized in the center of the telson region at the posterior end of the embryo (not found at the very outer region). nos mRNA is localized even further posterior than osk mRNA (at the very outer region), and it is localized slightly dorsally at the posterior tip. otd mRNA is highly concentrated within the future head region, and expressed at lower concentrations throughout the rest of the developing embryo; however, although otd mRNA is expressed at the outer part of the anterior region, it is not expressed in the outermost region anywhere else. hb mRNA is dispersed throughout the developing embryo, with the exception of a large middle portion of the future abdomen. In other words, hb mRNA is expressed at the anterior and posterior poles, but the anterior pole is more diffuse than the posterior pole. 1 PSI- ©BIOL463 2014 c) Propose a model that explains the functional/mechanistic interrelationships among the genes in question. Please do not worry about your model being “the right answer”. Instead, try to make a model that would fully explain the data shown here without contradicting any biology principle that you know. The gradients established by these gene products within the cytoplasm act as signals for the developing Drosophila embryo. These gradients are established in one region and spread throughout the rest of the embryo – whether by diffusion, oligimerization, or interaction with antagonist molecules. Assuming a steady synthesis rate, the diffusion rate and clearance rate help to determine the range of each morphogen. Both the combination and concentration of gene products within a region provide signals of differing type and intensity to the embryo. This model is known as the synthesis-diffusion-clearance model. As certain gene products appear to exclude others, it is possible that the presence of some gene products in Drosophila represses the presence of another gene product within that region. For example, Bcd protein and Cad protein are dispersed in the anterior and posterior regions of the embryo, respectively. It is possible that another protein, located in most of the future head and future thorax region, excludes the presence of either the Bcd or Cad proteins from presenting in within this region. Similarly, nos mRNA may repress the presence of osk mRNA – perhaps the product of osk mRNA is part of a signal transduction cascade which reduces the expression of membrane receptors needed to initiate transcription of osk mRNA. Similarly, high concentrations of otd mRNA appear to preclude the presence of bcd mRNA. Further, as bcd mRNA is more localized than the range of the Bcd morphogen, the Bcd morphogen likely diffuses into a larger range at the anterior region. hb mRNA may be more diffuse in the anterior region (compared with the posterior region) due to interactions with other molecules. For example, perhaps hb mRNA is synthesized at both the anterior and posterior poles, but a synergistic morphogen that upregulates hb mRNA expression is present in the anterior region and absent in the posterior region. Based on their locations, Bcd likely acts as a signal for development of the anterior region. Cad likely acts as a signal to induce development of the posterior region. d) How could you test your model (or a part of it)? Propose an appropriate experiment and predict the results that you would obtain if your model is accurate. I would choose to test whether a synergistic morphogen that could increase hb mRNA expression is present in the anterior cytoplasm but not the posterior cytoplasm. I would withdraw cytoplasm from the anterior region of a Drosophila embryo (which would include the gene products from that region), then administering this cytoplasm to the posterior region of a recipient Drosophila embryo. Assuming hb mRNA is synthesized at both the anterior and posterior poles of the embryo, the range of hb mRNA should be assessed. This would be compared to a wild type Drosophila embryo at the same stage in development. Results indicating an increased range of hb mRNA in the posterior region might suggest that a synergistic morphogen is present which increases the expression range of hb mRNA. On the other hand, negative results might indicate a different mechanism for the smaller range of hb mRNA in the posterior region (compared with the anterior region). 2 PSI- ©BIOL463 2014 Part II (in class). You will receive some additional relevant information so that you will be able to discuss and answer the following questions: e) In the early Drosophila embryo, what are the anterior and the posterior determinants, respectively? The major anterior determinant is Bicoid, and the major posterior determinant is the osk gene product. f) How could you test if your hypothesized determinants are truly determinants? Think about what experiment you’d do and what the prediction would be. Mutate the bcd and osk genes (in separate treatments) by targeted knock-out so they become homozygous loss-of-function mutations. Compare these treatments with control embryos (embryos with non-mutated genes). The phenotype of the embryos should be observed. If the anterior region develops abnormally for the bcd mutant, then the bcd gene is necessary for normal anterior region development. If the anterior region develops normally, then the bcd gene is not the anterior determinant. The same should be observed for the phenotype of the posterior region for the osk mutants. Further, if we added osk to the anterior region of a bcd knockout, we would expect two posterior regions to form. This would entail that the osk gene product is sufficient to induce the posterior region elsewhere. The same experiment should be conducted for the bcd gene product in a osk knockout, in which Bicoid would be added to the posterior region of a developing embryo and we would observe for the development of two anterior regions. This would tell us that the bcd gene is sufficient for anterior region development. g) Predict what happens in an embryo whose mother is homozygous for a complete knock-out of bcd. The Bicoid protein is necessary for normal anterior region development of Drosophila embryos. The posterior region would also develop abnormally, because the interactions between the bcd gene product with other proteins is important for normal development. Additionally, transcription of the otd and hb genes would not occur, because Bicoid activates their transcription. Cad protein might also be diffused more towards the anterior region, because Bicoid normally represses its presence in the anterior region. 2. swallow (swa) is a crucial maternal-effect gene in Drosophila. Weil and colleagues (2010) conducted a series of experiment to investigate its role(s) and possible mechanism of action. Here are some of the data they obtained: Genotype of Percentage of WT late oocytes female Drosophila with WT bcd mRNA localization swa+/swa+ (wild-type) 96% swaTN62/swaVA11 (heteroz, two mutant alleles) 2% TN62 TN62 swa /swa (homoz, same mutant allele) 10% Note: mutations in swa have no effect on the localization of bcd mRNA in early oocytes. 3 PSI- ©BIOL463 2014 a) Based on the data above, what do you conclude about the role/function of the swa gene product? The wild-type swa gene product is not necessary for females’ late oocytes to have localization of the bcd mRNA. However, the females’ swa gene product is involved in the localization of the bcd mRNA in late oocytes. b) Propose a hypothesis for the role of the Swallow protein in the localization of the bcd mRNA (if you can, include a molecular mechanism whereby Swallow might be carrying out its function). The Swallow protein localizes the bcd mRNA in late oocytes by interacting with the (+) microtubules at the anterior of the embryo, then tethering the mRNA to the anterior region. As this must occur in late, but not early, oocytes, Swallow is likely expressed in a temporally-restricted manner during late oocyte development. c) In 2010, Weil and colleagues performed a series of studies where they looked at the distribution of the Swallow protein and the bcd mRNA in Drosophila oocytes. Some of their results are shown in the figure below. What can be concluded from these results? bcd mRNA is found near the Swallow protein, but the Swallow protein is found without bcd mRNA in some cases. Specifically, Swallow and bcd mRNA do not colocalize in the anterior region of the developing embryo; it can be observed that Swallow and the bcd mRNA are spatially separated. Actual observations: Swallow is located in anterior region of the developing embryo. Complex (including bcd mRNA) interacts with microtubules and is brought to Swallow in the anterior region. When Swallow and the bcd mRNA interact, Swallow keeps the mRNA localized to the anterior region. 4 PSI- ©BIOL463 2014 This figure is from Weil et al. (2010). Please do not reproduce it or re-post it. A) and G) show the localization of GFP-tagged Swa protein in late oocytes and in the egg chamber, respectively. B) and H) show the bcd mRNA. C), I), J) and K) show both Swa and bcd. D) shows the localization of Stau, a protein that is essential for proper bcd localization, and F shows Stau (green) and the bcd mRNA. d) Do these results support or contradict your hypothesis proposed in b)? Somewhat. I incorrectly hypothesized that the Swallow protein moved along the microtubules; in reality, the Swallow protein remains at the anterior region of the embryo, while the bcd mRNA travels (as part of a complex) along microtubules towards the Swallow protein in the anterior region. 3. For the next question please refer to the diagrams and table below (from Berger et al, 2001). Again, please do not panic if you cannot answer all the questions or if there are some parts of the data that you are unsure about. You will have the opportunity to review and discuss your answer with your classmates. However, it is very important that you come to class having tried your best on it. Background information: The diagrams show parts of a fruitfly embryo at different stages of developments and the arrows summarize five different transplantation experiments that were carried out (I-V). Normally gastrulation (formation of ecto-, meso- and endoderm) is complete by stages 6-7; then the next big step is the differentiation of the ectoderm into either neuroectoderm or dorsal ectoderm: This is complete by stage 10. The cells that would normally give neuroectoderm are re presented in yellow (dorsal ectoderm in green). Then, during stages 10-16 the neuroectoderm cells further differentiate and acquire their ultimate cell identity. 5 PSI- ©BIOL463 2014 Note: you may disregard the distinction between clones from midline precursors and from neuroblaststhey are all neural clones) a) Interpret the data obtained from each of the transplantation experiments-what can we conclude from each one? (If you have trouble interpreting the table, do the best you can and ask for clarification in class). Transplantation I transplanted stage 10 neuroectoderm cells from one embryo into the same location on a stage 10 recipient embryo. This yielded roughly 50% neural and 50% epidermal clones. This acted as a control. This indicates that NE cells are not yet determined as neural or epidermal at stage 10. Transplantation II transplanted stage 10 neuroectoderm cells from one embryo into the same location on a stage 7 recipient embryo. This yielded about a 3:1 ratio of neural to ectodermal clones, indicating that repeating stages 7, 8, and 9 increase the likelihood of NE cells becoming neural clones. Transplantation III transplanted stage 7 DE cells from one embryo into the region of NE cells on a stage 7 recipient embryo. This yielded about a 2:1 ratio of neural to ectodermal clones. This indicates that stage 10 DE cells are not yet determined and increase their chance of becoming neural clones when transplanted into the NE. Transplantation IV transplanted stage 10 DE cells from one embryo into the region of NE cells on a stage 7 recipient embryo. This yielded only a few clones, with slightly more neural than ectodermal clones. Stage 10 DE cells are therefore not yet determined. This indicates that stage 10 cells may not be able to survive as well in the NE environment, which might indicate that stage 10 cells undergo regulated development at this point. This small sample size also means that our confidence for this conclusion is weak. Transplantation V transplanted stage 7 NE cells from one embryo into the NE region of a stage 10 recipient embryo. This yielded many epidermal clones, and less than 1/5 as many neural clones. This suggests that stages 8 and 9 are necessary for NE cells to become determined as 6 PSI- ©BIOL463 2014 neural clones. Perhaps stages 8 and 9 present a signal to the NE cells during these stages which tell the cells to become neurons. b) How could the researchers have tracked the descendants of the transplanted cells? Cells could have been radioactively labelled in order to track their location and development. For example, prior to insertion into the developing embryo, cells could be injected with a fluorescent transgene (such as GFP) with a constituently activated promoter. This would ensure that the GFP gene is always transcribed (at least at basal levels) and can therefore be detected using fluorescent microscopy. It would also be passed onto daughter cells. c) Are there any aspects of this experiment that could be improved? Make at least one suggestion. Transplantations II and IV appear to have been carried out simultaneously, and on the same recipient embryos. It is possible that these cells interacted differently with each other than they would have acted apart from each other. This introduces a variable that could be eliminated by performing transplantations II and IV separately. Sample size could have also been held more constant, and higher for Transplantation IV for more confidence. d) Are there any aspects of the data presentation that could be improved? Make at least one suggestion. I was initially confused with the labelling for the figure. I was unsure what the roman numerals stood for, and did not at first realize what the abbreviations stood for. A figure legend would clarify these problems. Statistical tests would also make the data clearer to understand. 7