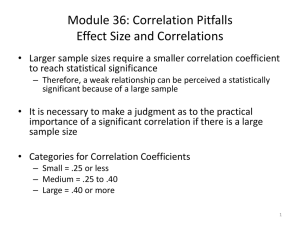
Data analysis - Proceedings of the Royal Society B
advertisement

1 Supporting Information 2 Data analysis 3 Testing for genetic variance in resistance and tolerance 4 We tested for genetic variance for resistance for both MA and non-MA lines by looking for an 5 effect of genotype on bacterial load. Genetic variance for resistance was assessed in each sex 6 separately using linear mixed-effect models of d, excluding the sham treatment, with initial load 7 as a fixed effect and genotype and block as random effects. We assessed the significance of fixed 8 effects using likelihood ratio tests, and the significance of random effects using the RLRsim 9 package [S1] which provides simulation-based exact restricted likelihood ratio tests for random 10 11 effects. Block was determined to be non-significant, and removed from the model. Genetic variance for tolerance would lead to an effect of genotype on the slope of the 12 relationship between relative fitness and bacterial load. We have two replicate measures of 13 tolerance within each genotype represented by the estimates of fitness and load at two infection 14 levels. We estimated genetic variance for tolerance in the presence of this residual variance in 15 each sex separately using linear mixed-effect models of w, with d as a fixed effect and genotype 16 as a random effect on the coefficient for d, with a fixed intercept of (0,1). We tested for 17 significance of random effects as for resistance, above. 18 19 Testing the resistance-tolerance correlation 20 The way in which we measured resistance and tolerance will lead to the appearance of a negative 21 genetic correlation between these estimates due to chance alone, in the absence of any true 22 underlying correlation. This spurious correlation arises because our resistance and tolerance 23 measures both involve bacterial load, d. To examine the true correlation between resistance and 24 tolerance we considered the traits that we measured independently: fitness and bacterial load. 25 Conceptually, in the absence of any true correlation between resistance and tolerance, excluding 26 the uninfected treatment, fitness and bacterial load will tend to be negatively correlated, 27 assuming that bacteria reduce fitness. A positive correlation between resistance and tolerance 28 will result in a correlation between fitness and load in infected flies that is more negative, since 29 genotypes with relatively high load will also tend to have relatively low fitness. The converse is 30 true when there is a negative correlation between resistance and tolerance: genotypes with 31 relatively high load will tend to have higher fitness than expected in the absence of any true 32 resistance-tolerance correlation, leading to a more positive correlation between fitness and load. 33 Using simulation, we were able to confirm these verbal arguments, and determine the 34 null distribution for the correlation between fitness and load in the absence of any true 35 correlation between resistance and tolerance. We determined the correlation between fitness and 36 load in our data, averaging across infection levels, and excluding the uninfected (sham) 37 treatment, by determining the expected values of w and d at a single arbitrary infection level, and 38 taking their rank correlation. We observed a slightly positive correlation between fitness and load 39 of 0.024 in males and 0.076 in females, suggesting a negative correlation between resistance and 40 tolerance in both sexes. We combined the evidence from both sexes by considering the average 41 fitness-load correlation across sexes, 0.050. (Note that this positive correlation does not imply 42 that bacteria increase fitness: uninfected values, which would make the fitness-load correlation 43 strongly negative, are excluded from this procedure). To determine whether this value is 44 expected by chance alone, given the distributions of resistance and tolerance that we observed, 45 we first determined the best-fit gamma distribution for our estimates of resistance and tolerance 46 in each sex using maximum likelihood (see details below). We then assigned random resistance 47 and tolerance values to 50 genotypes by simulating random values from these gamma 48 distributions. We then determined the resulting expected values of fitness and load for each 49 genotype and sex given these resistance and tolerance values, and determined the fitness-load 50 correlation, averaged across sexes. This procedure was repeated 10000 times. We confirmed that 51 the average simulated correlation between resistance and tolerance was approximately zero. We 52 found that the expected fitness-load correlation given random, uncorrelated values of resistance 53 and tolerance was approximately –0.156. The correlation we observed (0.050) was found to be 54 significantly more positive than expected given this null distribution (two-tailed P = 0.0340), 55 indicating that our data most likely reflect an underlying negative correlation between resistance 56 and tolerance (see also Fig. S3). In other words, the association we observed between fitness and 57 load is unlikely to have occurred in the absence of a true negative resistance-tolerance 58 correlation. 59 In addition to the procedure described above, we repeated this approach using 60 randomization of the association between the observed values of resistance and tolerance, rather 61 than simulating values from gamma distributions. The result was essentially the same: the 62 expected fitness-load correlation in the absence of a true resistance-tolerance correlation was - 63 0.171, and the correlation we observed was significantly more positive than this expected value 64 (two-tailed P = 0.0264). 65 66 Maximum likelihood details 67 We determined the most likely displaced gamma distribution (three parameters) for resistance 68 and tolerance in each sex, where the log likelihood was given by 69 log L(k, , | data) log DG(x i | k, ) i 70 where k and θ are shape and scale parameters, δ is the displacement, xi is a given data point 71 (line mean), and DG is the gamma density function. For each group we performed 72 optimization using the Nelder-Mead algorithm with the R function optim, replicated 20 times 73 with different random starting parameters, and determined the best parameter values. Most runs 74 converged on similar values, but for male resistance we performed an additional 20 optimization 75 runs to ensure we found the best parameters. The best-fit parameters are shown in Table S1, and 76 were found to be a good fit to the actual data by visual inspection. 77 78 Supplemental References 79 S1. Scheipl, F., Greven, S., and Kuechenhoff, H. (2008). Size and power of tests for a zero 80 random effect variance or polynomial regression in additive and linear mixed models. 81 Computational Statistics & Data Analysis. 52, 3283-3299. 82 Table S1. Estimates of genetic variance (VG) for resistance and tolerance in mutant and non-mutant males and 83 females; residual variance (Vresid); number of replicates (N); number of genotypes (n); restricted likelihood ratio 84 (RLR); p-value (p). Trait Sex Group N n VG Vresid RLR p Resistance Male Non-mutant 200 25 0.1481 1.8127 3.0267 0.0349 Resistance Male Mutant 200 25 0.2086 2.2132 3.8305 0.0223 Resistance Female Non-mutant 200 25 0.1235 1.2094 4.3647 0.0152 Resistance Female Mutant 201 25 0.3040 1.6933 10.4528 0.0004 Tolerance Male Non-mutant 72 24 0.0066 0.7805 2.1386 0.0662 Tolerance Male Mutant 69 23 0.0237 0.7047 8.4176 0.0019 Tolerance Female Non-mutant 69 23 0.0021 0.0781 9.2959 0.0012 Tolerance Female Mutant 66 22 0.0011 0.0858 3.4652 0.0292 85 86 87 88 Table S2. Best-fit parameters from maximum likelihood models of resistance and tolerance in each sex. Sex Trait Shape Scale Displacement Male Resistance 1.0368 0.0525 -0.3625 Female Resistance 2.4168 0.0244 -0.3383 Male Tolerance 1.2846 0.1109 0.1931 Female Tolerance 4.4249 0.0249 0.2206 89 Figure S1. Details of fly crosses performed. The first three chromosomes are shown for each 90 genotype; the tiny fourth chromosome was not manipulated. Males lack recombination, and are 91 identified here by the presence of a Y chromosome. Crosses took place using virgin females 92 where appropriate. Chromosomes were identified using recessive phenotypic markers (bw, vg, 93 se), dominant phenotypic markers (L, Ki), and a balancer chromosome (CyO), which suppresses 94 recombination on the second chromosome. Mutation accumulation (MA) details are given in ref. 95 [25]. Briefly, a single second chromosome marked with bw was used to initiate three control 96 populations and numerous MA lines. These focal chromosomes are shown in red. (A) Control 97 populations homozygous for the focal chromosome were maintained at a moderate size (450 98 adults) to prevent mutation accumulation. (B) MA chromosomes were propagated by 99 bottlenecking to a single heterozygous male each generation, allowing new mutations to 100 accumulate. (C) Following 62 generations of MA, crosses were performed to replace all non- 101 focal chromosomes with an isogenic background. Within-line variation on the focal chromosome 102 was eliminated by bottlenecking (square brackets). Each cross included 1-4 males and 4 females 103 per line. These crosses involved several marker stocks, which were created using standard 104 crossing methods (not shown). An isogenic stock with vgL/CyO was created as shown in (D), 105 after creating a completely isogenic genotype using standard balancer chromosome methods (not 106 shown). We obtained focal males (E) and females (F) from 25 control lines and 25 MA lines. 107 Focal flies were inoculated with sterile MgSO4 or one of two doses of P. aeruginosa. One day 108 following inoculation flies were either homogenized and plated to assess bacterial load or placed 109 in mating groups to assess fitness. Each replicate mating assay consisted of one focal fly, one 110 wild-type competitor of the same sex, and two outbred bw/bw flies of the opposite sex. Flies 111 were allowed to interact and oviposit for three days, and then discarded. Offspring phenotypes 112 were scored 12 and 15 days after the start of the mating trial. We measured absolute fitness as 113 the number of bw/bw offspring relative to the total number of offspring produced over three 114 days. 115 116 Figure S2. Approach to estimating resistance and tolerance. Example data are shown to 117 illustrate how resistance and tolerance were estimated. (A) We operationally defined resistance 118 as the ability to limit the growth of bacteria. For each combination of genotype, sex, and dose we 119 first calculated mean bacterial load across i replicates. Mean bacterial load was calculated as d = 120 E[log10(coli + 1)], where coli is the number of colonies scored in replicate i. Resistance was 121 calculated as 1/mR, where mR is the linear slope of d on initial dose (dashed line), with a fixed 122 intercept of (0,0) (i.e., when initial load is zero, bacterial load is always zero). Transforming the 123 slope as –mR instead of 1/mR produced the same results. Initial loads were constant across 124 groups, represented by 0, 1.85, and 2.75. (B) We operationally defined tolerance as the ability to 125 maintain fitness in the presence of bacterial load, relative to fitness in the absence of bacteria. 126 For each combination of genotype, sex, and infection level we first calculated mean fitness 127 across replicates as W = Σbwi/ Σtotali, where bwi and totali are the number of brown-eyed 128 offspring and the total number of offspring scored in replicate i, respectively. Relative fitness at 129 infection level k was then calculated as wk = Wk/W0, where W0 is fitness in the absence of 130 infection (sham treatment, i.e. injection with sterile MgSO4). Tolerance was calculated as the 131 linear slope of w on d (dashed line, mT), with a fixed intercept of (1,0) (i.e., sham-treated flies, 132 with d = 0, have relative fitness of 1), where d is our estimate of mean bacterial load for that 133 combination of genotype, sex, and infection level, as described in (A). 134 135 Figure S3. Results of simulations to test the resistance-tolerance correlation. Each point 136 represents the correlation between simulated resistance and tolerance values, averaged across 137 sexes (x), and the resulting fitness-load correlation, averaged across sexes (y). The average 138 simulated resistance-tolerance correlation was approximately zero (vertical gray line). The 139 fitness-load correlation expected given this null distribution was approximately -0.156 140 (horizontal dashed line). The fitness-load correlation we observed (0.050, horizontal solid line), 141 was significantly more positive than expected under the null distribution (P = 0.0340). This 142 figure also illustrates that lower resistance-tolerance correlations are associated with higher 143 fitness-load correlations.