IACUC (Ethical Committee) and Animal Use Protocol
advertisement

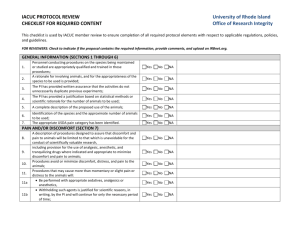
Other Required Information Answer ALL questions below. 1. Are you seeking financial support from other sponsors (including other J&J companies) (Yes/No)? 2. Are you a Medical Education and Communications Company (MECC) (Yes/No)? 3. Does your organization require an internal review/approval prior to this application being submitted (Yes/No)? If “YES”, has this review/approval been completed Yes/No? 4. Accreditation Statement (for CE/CME requests only) THIS IS A SAMPLE ACCREDITATION STATEMENT. ENTER YOUR OWN IN THE SPACE PROVIDED BELOW. “The (name of accredited provider) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The (name of accredited provider) designates this educational activity for a maximum of XX AMA PRA category 1 credits ™ Enter your Accreditation Statement in the space provided below: 5. Lab - Does your program include a LIVE ANIMAL LAB? - Does your program include a HUMAN CADAVER LAB? Lab Site: USDA # AAALAC # - If you are running a lab, please indicate the setting for the lab: ☐ Conventional Setting: Those settings where animal or cadaver laboratories are routinely performed. These settings would include universities/colleges, contract research organizations, and teaching hospitals. ☐ Nonconventional Setting: Those settings where animal and cadaver laboratories are not routinely performed. These settings would include hotel meeting spaces, convention centers, and mobile units. If you answered YES to HUMAN CADAVER LAB, the grant requestor agrees that it is in compliance with the following parameters in order to determine support of cadaver training laboratories in nonconventional settings: ☐ An authorized party at the non-conventional location has granted permission to run an animal or cadaver lab at that location. ☐ Access to the lab area will be controlled so that only individuals who are registered for the lab or who have been otherwise approved by the instructor will be allowed access. The doors to the lab area will remain locked at all times. ☐ The lab area is separate from public areas and cadaver entry/removal in and out of building is discrete. ☐ The lab area will be cleaned after the training is completed. ☐ Lawful and informed written consent has been obtained of the donors or individuals having authority under applicable state law to consent to the donation. All personal and medical information relating to the anatomic specimens and their donors shall remain confidential except as necessary to ensure the safety of individuals that come in contact with the specimens. ☐ (i) Documented processes are in place regarding the sourcing, transportation, handling, use, and disposition of anatomic specimens which are in compliance with all applicable laws and regulations, and (ii) all necessary permits, licenses and approvals required under such laws and regulations have been obtained and maintained. ☐ All personnel handling the anatomic specimens are trained and will comply with all applicable standards for protection from blood-borne pathogens. ☐ The disposition of all anatomic specimens complies with all applicable laws and is in accordance with the informed consent given by the donor and/or the donor’s legally authorized representative. ☐ All anatomic specimens will be treated with dignity and respect. If you answered YES to LIVE ANIMAL LAB, the following documentation must be submitted for review in order to determine support of live animal training laboratories in nonconventional settings: ☐ Completed “Checklist for Laboratory Animal Welfare” (BELOW) ☐ Copy of your IACUC approved protocol with documentation of the date of approval ☐ Approval date documentation can be contained on the protocol or can be separate from the protocol in the form an approval letter that references the IACUC protocol number ☐ Copy of your USDA registration ☐ Copy of the veterinary licensure of the onsite veterinarian ☐ Acknowledgement of the AAALAC, International status. (Please note this certification is preferred but not required.) This Animal Welfare Checklist is provided to assist in ensuring compliance with all applicable laws, regulations, and policies. This checklist summarizes the basic requirements for performing live animal training laboratories sponsored by the Global Education Solutions organization of J&J. The checklist is based on the following documents: 1. Animal Welfare Act – administered by the United States Department of Agriculture http://www.aphis.usda.gov/animal_welfare/downloads/awr/awr.pdf http://www.aphis.usda.gov/animal_welfare/downloads/awa/awa.pdf 2. Animal and Plant Health Inspection Service/Animal Care Policy Manual http://www.aphis.usda.gov/animal_welfare/policy.php 3. Guide for the Care and Use of Laboratory Animals, 8th Edition http://www.aaalac.org/resources/theguide.cfm 4. Johnson and Johnson Policies http://www.jnj.com/wps/wcm/connect/dd12c9804f5568229fc6bf1bb31559c7/guidelines-for-the-use-of-animals.pdf?MOD=AJPERES http://www.jnj.com/wps/wcm/connect/06f78a004fbeff879356b753d1df9354/policy-on-the-humane-care-new.pdf?MOD=AJPERES 5. 2013 AVMA Guidelines on Euthanasia https://www.avma.org/KB/Policies/Documents/euthanasia.pdf Contractors are also responsible for assuring compliance with any relevant state or local regulations. CHECKLIST FOR LABORATORY ANIMAL WELFARE Requirements IACUC (Ethical Committee) and Animal Use Protocol There is a functional IACUC of record at or for the facility and its structure and function is compliant with the Animal Welfare Regulations. The contractor’s IACUC has reviewed and approved the animal use for the training laboratory. The animal use protocol must accurately describe all the procedures being performed. The animal use protocol must contain all appropriate information required for regulatory compliance. A copy of the approved protocol (hard copy or easily accessible online) is available to all laboratory personnel and personnel are aware of the procedures listed on the approved protocol. Laboratory personnel understand that the only procedures that can be conducted without a protocol modification are those listed. All personnel providing any animal care/use are listed on the approved protocol. There are procedures in place for internal and external allegations of improper animal care and use to be promptly investigated by the IACUC. Personnel Qualifications & Training All personnel involved with the laboratories are trained to the level of their positions. Personnel training is provided as is appropriate for the species and/or hazardous agent(s) used (e.g., zoonoses, hazards, special precautions). Animal Procurement & Transportation All animals are obtained lawfully and transportation is consistent with USDA regulations and humane practices. Animal shipments are inspected for compliance with procurement specifications and signs of clinical disease. The ordering and receiving of animals is coordinated with the appropriate animal care personnel to assure that the appropriate facilities are available for use. Yes No Veterinary Medical Care The Veterinarian is licensed in the US and has experience with the species being used. The Veterinarian has authority to provide medical treatment at his/her discretion. The Veterinarian can access all animals and animal procedure areas as needed. The Veterinarian provides guidance on handling, sedating, anesthetizing, and euthanizing of animals. Pain, Distress, Analgesia & Anesthesia Personnel caring for and using animals are trained in and are responsible for assessing pain and distress in the animals. Compliant drug control and storage procedures are in place, including secure storage and tracking of controlled substances. Anesthetics, analgesics, and euthanasia drugs are never used past their expiration date. Only the specific drugs, methods, and materials approved in the protocol may be used on the animals unless written documentation is provided from a veterinarian permitting and justifying their use. Nonsurvival Surgery/Procedures Anesthesia must be monitored regularly and adjusted as needed to maintain an appropriate depth of anesthesia. At a minimum, the surgical site should be clipped, the surgeon should wear gloves, and instruments and the surrounding area should be clean. Euthanasia Euthanasia methods are consistent with the AVMA recommendations and are appropriate to the species. The euthanasia method must match that approved by the IACUC. All relevant individuals are trained in the method of euthanasia to be used. Death must be confirmed in all euthanized animals before disposal. Carcass/Waste Disposal All carcass/waste disposal meets facility, municipal, and federal policies and regulations. Conventional, biological and hazardous waste is removed and disposed of safely. Physical Plan and Safety There is no food or drink in proximity to the areas where the animals are used. Procedures are in place to provide appropriate personal protective equipment such as masks, respirators, gowns, eye protections, boots, etc. Gloves and protective clothing must be used at all time when handling animals/performing surgery/performing procedures. Appropriate security measures are implemented. Compressed gas cylinders, if used in procedural areas are properly immobilized. Plans for facility emergencies are available for implementation if required. 6. REQUEST FOR TECHNICAL SUPPORT ON-SITE? (Yes/No)? 7. Product Shipping Information (REQUIRED for grants requesting product) DELIVER BY DATE: _____________________________________________________ Ship To: Attention: Department: Institution Name: Address 1: Address 2: City, State Zip: Phone Number: 8. Product Request List (next page) Please use these sites to find product codes: Http://ecatalog.ethicon.com/ http://www.acclarent.com/solutions/products/product-catalog/ http://www.ees.com/sites/default/files/catalog.pdf http://www.mentorwwllc.com/global-us/AllProducts.aspx Prod. Code Quantity Requested 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Add additional rows as required Product Description