Supplemental Figure 1. Supplemental Table 1. Footnotes to Figure 1
advertisement

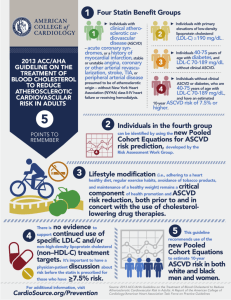
Supplemental Figure 1. 1 Supplemental Table 1. Footnotes to Figure 1. Initiation of statin therapy Summary of Statin Initiation Recommendations for the Treatment of Blood Cholesterol to Reduce ASCVD Risk in Adults (See Figures Figure 3, Figure 4, and Figure 5 for More Detailed Management Information in the 2013 ACC/AHA cholesterol guideline report) *Percent reduction in LDL-C can be used as an indication of response and adherence to therapy, but is not in itself a treatment goal. †The Pooled Cohort Equations can be used to estimate 10-year ASCVD risk in individuals with and without diabetes. The estimator within this application should be used to inform decision making in primary prevention patients not on a statin. ‡Consider moderate-intensity statin as more appropriate in low-risk individuals. §For those in whom a risk assessment is uncertain, consider factors such as primary LDL-C ≥160 mg/dL or other evidence of genetic hyperlipidemias, family history of premature ASCVD with onset <55 years of age in a first-degree male relative or <65 years of age in a first-degree female relative, hs-CRP ≥2 mg/L, CAC score ≥300 Agatston units, or ≥75th percentile for age, sex, and ethnicity (for additional information, see http://www.mesanhlbi.org/CACReference.aspx), ABI <0.9, or lifetime risk of ASCVD. Additional factors that may aid in individual risk assessment may be identified in the future. ‖Potential ASCVD risk-reduction benefits. The absolute reduction in ASCVD events from moderate- or high-intensity statin therapy can be approximated by multiplying the estimated 10-year ASCVD risk by the anticipated relative-risk reduction from the intensity of statin initiated (∼30% for moderate-intensity statin or ∼45% for high-intensity statin therapy). The net ASCVD risk-reduction benefit is estimated from the number of potential ASCVD events prevented with a statin, compared to the number of potential excess adverse effects. ¶Potential adverse effects. The excess risk of diabetes is the main consideration in ∼0.1 excess cases per 100 individuals treated with a moderate-intensity statin for 1 year and ∼0.3 excess cases per 100 individuals treated with a high-intensity statin for 1 year. In RCTs, both statin-treated and placebo-treated participants experienced the same rate of muscle symptoms. The actual rate of statin-related muscle symptoms in the clinical population is unclear. Muscle symptoms attributed to statin therapy should be evaluated (see Table 8, Safety Recommendation 8 in the 2013 ACC/AHA cholesterol guideline report). 2 ABI indicates ankle-brachial index; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; and RCT, randomized controlled trial. 3 Supplemental Table 2. Case examples for Table 1. Examples: 1. Very high risk patient with LDL-C <100 mg/dl (2.59 mmol/L). Average patient within 10 days of acute coronary syndrome with additional high risk factor in IMPROVE-IT [1]: 7-year event rates: Simvastatin+placebo group had 22.2% ASCVD event rate (3.2% per year) with mean LDL-C 70 mg/dl (1.81 mmol/L) versus Simvastatin + Ezetimibe group had 20.4% ASCVD event rate and median achieved LDL-C 54 mg/dl (1.40 mmol/L); RRR 10% in ASCVD events Clinical application: Absolute CVD risk of about 15% over 5-years OR 30% over 10 years Relative risk reduction of 10% from about an 18 mg/dl (0.47 mmol/L) reduction in LDL-C (LDL-C 7052 mg/dl or 8870 mg/dl) (LDL-C 1.811.35 mmol/L or 2.281.81 mmol/L) 5-year NNT67 OR 10-year NNT33 Reasonable to consider addition of ezetimibe if patient otherwise likely to have a good quality life expectancy for 5 years or more 2. High risk patient with LDL-C <100 mg/dl (2.59 mmol/L). Average patient with chronic CHD in TNT[2]: Mean follow-up 5 years; Atorvastatin 10 mg group had 10.9% 5-year hard ASCVD event rate with mean LDL-C 98 mg/dl (2.54 mmol/L) versus atorvastatin 80 mg group had 8.7% 5-year hard ASCVD event rate with mean LDL-C 77 mg/dl (2.0 mmol/L). Clinical application: Absolute CVD risk of about 10% over 5-years OR 20% over 10 years 4 Relative risk reduction of 15% from about an 18 mg/dl (0.47 mmol/L) reduction in LDL-C (LDL-C 7052 mg/dl or 8870 mg/dl) (LDL-C 1.811.35 mmol/L or 2.281.81 mmol/L) 5-year NNT100 OR 10-year NNT50 Clinician and patient should discuss whether this is a meaningful clinical benefit over 5-10 year treatment period 3. High risk patient with LDL-C >130 mg/dl (3.37 mmol/L). Average patient with chronic CHD (20% 10year ASCVD risk) and LDL-C 130 mg/dl (3.37 mmol/L) on maximally tolerated statin therapy. Clinical application: Absolute CVD risk of about 10% over 5-years OR 20% over 10 years Relative risk reduction of 10% from about a 26 mg/dl (0.67 mmol/L) reduction in LDL-C (LDL-C 130 →104 mg/dl)(LDL-C 3.372.69 mmol/L) 5-year NNT 67 OR 10-year NNT33 Reasonable to consider addition of ezetimibe if patient otherwise likely to have a good quality life expectancy for 5 years or more 4. Patient with genetic hypercholesterolemia (untreated LDL-C >190 mg/dl; 4.92 mmol/L). All patients with genetic hypercholesterolemia should be treated with a high intensity statin if possible, and a >50% reduction in LDL-C is desirable. Do not estimate 10-year ASCVD risk conventional risk prediction equations in patients with genetic hypercholesterolemia because they do not capture the high risk of premature ASCVD in these patients. Exposure to severe hypercholesterolemia has been present since 5 birth and lifetime risk of ASCVD is very high in patients with genetic hypercholesterolemia, therefore the addition of ezetimibe would be reasonable for many of these patients. PCSK-9 inhibitors may be particularly promising in patients with genetic hypercholesterolemia if they are shown to reduce ASCVD outcomes and are safe in the long-term outcomes trials. These drugs can lower LDL-C 50-65% when added to background lipid-lowering therapy. [3,4]. References 1. Cannon C, On behalf of the IMPROVE-IT study group. IMProved Reduction of OUtcomes: Vytorin Efficacy International Trial. Presented at the American Heart Association Scientific Sessions, November 17, 2014 [serial on the Internet]. 2014 2. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart J-C, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-35. 3. Raal F, Scott R, Somaratne R, et al. Low-Density Lipoprotein Cholesterol–Lowering Effects of AMG 145, a Monoclonal Antibody to Proprotein Convertase Subtilisin/Kexin Type 9 Serine Protease in Patients With Heterozygous Familial Hypercholesterolemia: The Reduction of LDL-C with PSCK-9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) Randomized Trial. Circulation. 2012;126:2408-2417. 4. Robinson J, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Long-term safety, tolerability and efficay of alirocumab versus placebo in high cardiovascular risk patients: first results from the ODYSSEY LONG TERM study in 2,341 patients. In: Hot Topic, editor. European Society of Cardiology Congress; August 31, 2014; Barcelona, Spain2014. CHD: Coronary heart disease CVD: Cardiovascular disease 6 ASCVD: Atherosclerotic cardiovascular disease (hard events defined as myocardial infarction, stroke, cardiovascular death) NNT: Number-needed-to-treat TNT = Treating to New Targets 7