DDB Research Information Release Policy
advertisement

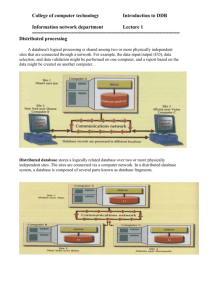
Release of Information to Researchers The DDB regularly receives requests from external researchers for data or information to assist with research projects. Such requests may vary from a simple request for some statistics to more complex requests such as identifying DDB clients who fit specific research criteria. In principle the DDB tries to be as cooperative as possible with bona fide researchers to promote scientific research in the public interest, particularly in the area of occupational dust disease. The DDB Privacy Disclosure Statement advises all applicants that the DDB may use their personal and health information for DDB approved medical research purposes. Release of DDB client information is subject to the Statutory Guidelines on Research relating to the Health Records and Information Privacy Act 2002 (NSW) governing the release of private information for research in the public interest. Incoming research requests to the DDB are directed to the Manager Research and Education, or the Director Medical Services, or the Right to Information Officer, or the General Manager. Research requests fall into four categories: 1. De-identified statistics 2. Mail-out to DDB clients 3. Identifiable client/employer information 4. Direct access to DDB records Category 1 requests are usually received via email. The Director Medical Services is delegated to release information of this type. Considerations for approval of these requests include: Credentials/institutional affiliation of the researcher requesting information Potential sensitivity of the information The amount of time and resources required to respond to the request Whether data aggregation is sufficient to prevent individual identification. Potentially sensitive information must be approved by the General Manager before release. Category 2 requests come from researchers seeking DDB assistance to identify suitable DDB clients who may be prepared to participate as research subjects for a project. Such requests must come as written requests including a draft research protocol. These requests usually require a meeting with the Director Medical Services and the Manager Research & Education to discuss the project and whether it is possible to match the research criteria with DDB client data. DDB collaboration in this type of project involves: Identifying individual clients from the DDB database Working with the researcher to ensure that the cover letter to clients is acceptable to the DDB Enveloping and posting the letters to clients. The DDB does not disclose the names or addresses of our clients to the researcher. The cover letter always goes on DDB letterhead. The offer to participate in the research project always reassures the client that their decision to participate or not will have no effect on their relationship with the DDB or their compensation entitlement from the DDB. The clients contact the researcher directly if they wish to participate as a research subject. The General Manager is delegated to approve such research collaboration. Before providing this type of information it is a requirement that the researcher provides to the DDB a copy of the project approval by an institutional ethics committee. Considerations for approval of these requests include: Credentials/institutional affiliation of the researcher The number of clients in the mail-out The amount of time and resources required to comply with the request Any potential to undermine DDB determinations or DDB reputation Category 3 & 4 requests relate to projects where client identification is unavoidable, or is required so that the researchers can obtain further medical records from treating doctors/hospitals. This type of project requires approval from an institutional ethics committee. The General Manager is delegated to approve such research collaboration. Before providing this type of information it is a requirement that the researcher provides to the DDB a copy of the project approval by an institutional ethics committee. Considerations for approval of these requests include: Signed confidentiality agreement Credentials/institutional affiliation of the researcher Guarantees that all identifying information will be stored securely Guarantees that no individual client will be identifiable in published results The amount of time and resources required to comply with the request Any potential to undermine DDB determinations or DDB reputation Endorsed by the Dust Diseases Board on 17 July 2014.