Click here

Julio ___
Joey ___
Mrs. Hochberg
10/07/13
Period #9
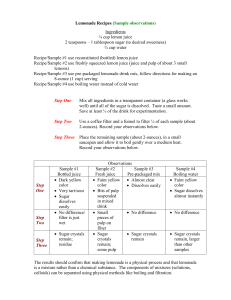
Analysis of Density
Introduction:
Do you know how much sugar is in your drink? This question is asked because nutritionists have recently raised concerns about increasing popularity of sodas, fruit drinks, and other beverages due to their high sugar content. The objective of this lab is to identity the amount of sugar in a drink based on its density. The density of a solution can be best determined by a graph called the calibration curve. If Coca-Cola has the highest density value, then it will also have the highest sugar content.
Materials:
Beverages at room temperature
Welch’s grape juice
Lemon lime powerade
Coca-Cola
Mott’s apple juice
25mL graduated cylinder
250mL beakers
Thermometer
Balance (0.01g precision)
Procedure:
1)
Place a clean 25mL graduated cylinder on a balance and press “tare” or “zero”
2) Remove the cylinder off the balance, and fill the cylinder to the 10.0 mL mark with a beverage. Measure and record the mass of the beverage in the graduated cylinder in your data table.
3) Measure and record the temperature of the beverage.
4) Re-zero the balance with the graduated cylinder containing 10.0mL of your beverage.
Remove the cylinder off the balance and fill the graduated cylinder to the 20.0mL mark with the same beverage. Measure and record the mass of the second sample.
5) Lastly, calculate and record the density of each beverage sample, then determine the average density off the beverage solution.
Beverages
Data:
Mass
Coca-Cola 9.95g
Welch’s grape juice
10.06g
Mott’s apple juice
Powerade
0.99g
0.96g
Volume
10mL
10mL
1mL
1mL
Density
.995 g/mL
Average Density
1.01g/mL
Temperature
22 °C
1.006 g/mL
0.99 g/mL
1.038g/mL
0.99g/mL
0.96 g/mL 0.99g/mL
% sugar 1% 5% 10% 15% 20%
Density at
20 °C
1.002 g/mL 1.018 g/mL 1.038 g/mL
Data Analysis / Calculations:
Density = mass / volume
Density of coca cola =9.95 g / 10 mL = .995 g/mL
Density of grape juice = 10.6g / 10 mL = 1.006g g/mL
Density of Apple juice = 0.99 g/1 mL = .99 g/mL
Density of Powerade = 0.96 g / 1 mL = 0.96 g/mL
1.059 g/mL
1.081 g/mL
22 °C
22 °C
22 °C
Coca-Cola:
Avg density-1.01g/mL
Nutrition label -55 g per 500mL (55/500) =0.11g
Percent sugar-0.11g *100%= 11%
Welch’s Grape juice:
Avg density -1.038g/mL
Nutrition label- 36g per 240mL (36/240) = 0.15
Percent sugar- 0.15 *100% = 15%
Mott’s apple juice:
Avg density- 0.99g/mL
Nutrition label- 28g per 240mL (28/240) = 0.16
Percent sugar- 0.16 *100% =16%
Powerade:
Avg density- 0.99g/mL
Nutrition label- 21g per 360mL (21/360) =0.058
Percent sugar- 0.0058*100%=58%
Conclusion:
This lab turned out to be easy and hard at the same time. There was a few times were calculations were not accurate. Therefore this lab had a few errors as the PowerAde sugar percentage was too high. The whole reason for this lab was to measure the densities of popular beverages, and determine their sugar contents using a calibration curve obtained by plotting the densities for a series of reference solutions versus percent sugar. The hypothesis was that coca cola has the highest percentage of sugar, this was proven incorrect.