Lab 11
advertisement

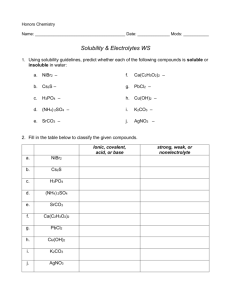
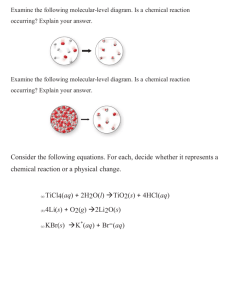
Lab 4.1 Precipitation Reactions Introduction The majority of ionic solids are soluble in water. Those that are not account for the observation that solid products, called precipitates, are sometimes formed when aqueous ionic solutions are mixed. Ionic compounds are made up of positive and negative ions held together by the attractive, electrostatic forces between the oppositely charged particles. When soluble ionic compounds are placed in water they break apart to give separate ions. The solid dissolves. When two ionic solutions are combined, the resulting mixture contains positive and negative ions from each solution. The mixing permits new combinations of ions and if one or more of these new ion combinations happens to be insoluble in water, it “falls out” of solution as a solid compound. The insoluble product formed in this way is called a precipitate. As an example, consider the addition of a solution of sodium chloride to a solution of silver nitrate. The sodium chloride solution contains sodium ions, Na+, and chloride ions, Cl-. The silver nitrate solution contains silver ions, Ag +, and nitrate ions, NO3-. The mixture will contain all four ions and two new ion combinations will be possible: Ag+ with Cl- and Na+ with NO3-. The 2 possibilities are: Na+(aq) + NO3-(aq) NaNO3-(s) or: Ag+(aq) + Cl-(aq) AgCl(s) The ionic compound NaNO3 is soluble in water so no precipitate will result from the combination of Na + and NO3-. The ionic compound AgCl, however, is not soluble in water and therefore, the Ag+ and Cl- will leave the solution as a precipitate. This particular reaction may be represented by the complete ionic equation: Na+(aq) + Cl-(aq) + Ag+(aq) + NO3-(aq) Na+(aq) + NO3-(aq) + AgCl(s) It shows the ions that are present, even those that are not involved in the precipitation reaction. The ions that do not precipitate in the reaction, in this case Na+ and NO3-, are called spectator ions. An equation that omits the spectator ions is called a net ionic equation. The net ionic equation for the reaction that occurs when the sodium chloride and silver nitrate solutions are mixed is as follows: net ionic equation: Ag+(aq) + Cl-(aq) AgCl(s) The symbol (s) after AgCl indicates that this compound is a solid. Sometimes (s) is replaced by an arrow pointing down to show that the compound is a solid that will precipitate out of the solution. The symbol (aq) indicates that the associated ion is present in aqueous solution – it is dissolved in water. In this experiment, you will mix six different ionic solutions in all possible combinations of two, to determine which combinations result in precipitate formation. Based on your results, you will write complete and net ionic equations for each reaction that has taken place. 1. Wear safety goggles. 2. Potassium chromate and sodium chromate are corrosive substances and can cause sever skin or eye injury. They are also toxic. If you spill these materials on yourself, immediately flush the affected area with water and notify the teacher. 3. Aluminum chloride is an irritant. Avoid skin contact with this chemical. 4. Silver and barium compounds are poisonous. Avoid direct contact with these chemicals and wash your hands thoroughly after use. 5. Silver nitrate will leave dark brown stains on skin and clothing. These stains will disappear from the skin in time, as the stained skin cells flake off, but the stains will not come out of clothing. Be careful not to spill this solution. 6. Sodium hydroxide is a very caustic material that can cause severe skin burns. Eye burns caused by sodium hydroxide are progressive; what at first appears to be a minor irritation can develop into a severe injury unless the chemical is completely flushed from the eye. If sodium hydroxide is gotten in the eye, flush the eye with running water for at least 15 minutes. Notify the teacher immediately. If sodium hydroxide is spilled on another area of the body, flush the affected area continuously with running water for a minimum of 10 minutes. Objectives 1. To determine which combinations of ionic solutions form precipitates. 2. To identify the precipitate in each reaction. 3. To write complete and net ionic equations for each reaction. Procedure 1. Obtain a spot plate, a set of chemicals in dropper bottles, a 600mL waste beaker, and a washer bottle. 2. Mix each pair of solutions listed in the data table in separate wells on the spot plate. Use two drops of each solution. Be careful not to contaminate the dropper from one bottle with a different solution. In other words, DONT DIP THE TIPS INTO THE WELLS! AND DONT MIX THE CAPS! 3. Record the results of this experiment in the data table. Make as many observations as possible during the experiment, including the color of any precipitate formed. Be prepared! Not very many of these solutions will form precipitates! 4. Each time the wells of the spot plate are filled, discard the used chemicals into the waste beaker, rinse out the wells with distilled water, and continue. The wells do not need to be dried. 5. Clean your work area and wash your hands thoroughly before leaving the laboratory. Data Analysis 1. For each combination of solutions that gave a precipitate, there were two possibilities. Write correct formulas for the two new compounds that could form from the ions present. Use the example from the introduction to help you. Enter these formulas in the data table. 2. Decide which of the two new compounds is the precipitate by eliminating non-possibilities. Remember these two hints: (a) All the chemicals in your set are soluble, and so cannot be precipitates in any of the reactions. (b) A combination of ions will either always form a precipitate or never form one. If you suspect that a particular compound is the precipitate, check to see if that compound was also formed in another test. 3. Write the net ionic equations for all the reactions that formed precipitates, at the bottom section of your data table. 4. Explain in your words and with a set of diagrams why and how a precipitate forms. Data Table – Tests for Precipitate Formation (Broken up into 3 sets, hopefully to help you keep order to this madness!) Solution A Solution B Precipitate Formed? Color? Formulas for 2 possible precipitates (Only trials where a ppt formed) 1 Ba(NO3)2 2 Na2SO4 1 Ba(NO3)2 3 Al2(SO4)3 1 Ba(NO3)2 4 Mg(NO3)2 1 Ba(NO3)2 5 MgCl2 1 Ba(NO3)2 6 AlCl3 2 Na2SO4 3 Al2(SO4)3 2 Na2SO4 4 Mg(NO3)2 2 Na2SO4 5 MgCl2 2 Na2SO4 6 AlCl3 3 Al2(SO4)3 4 Mg(NO3)2 3 Al2(SO4)3 5 MgCl2 3 Al2(SO4)3 6 AlCl3 4 Mg(NO3)2 5 MgCl2 4 Mg(NO3)2 6 AlCl3 5 MgCl2 6 AlCl3 1 KCl 2 MgCl2 1 KCl 3 Na2SO4 1 KCl 4 NaOH 1 KCl 5 BaCl2 1 KCl 6 MgSO4 2 MgCl2 3 Na2SO4 2 MgCl2 4 NaOH 2 MgCl2 5 BaCl2 2 MgCl2 6 MgSO4 3 Na2SO4 4 NaOH 3 Na2SO4 5 BaCl2 3 Na2SO4 6 MgSO4 4 NaOH 5 BaCl2 4 NaOH 6 MgSO4 5 BaCl2 6 MgSO4 1 BaCl2 2 Mg(NO3)2 1 BaCl2 3 Na2CrO4 1 BaCl2 4 Al2(SO4)3 1 BaCl2 5 K2CrO4 1 BaCl2 6 AgNO3 2 Mg(NO3)2 3 Na2CrO4 2 Mg(NO3)2 4 Al2(SO4)3 2 Mg(NO3)2 5 K2CrO4 2 Mg(NO3)2 6 AgNO3 3 Na2CrO4 4 Al2(SO4)3 3 Na2CrO4 5 K2CrO4 3 Na2CrO4 6 AgNO3 4 Al2(SO4)3 5 K2CrO4 4 Al2(SO4)3 6 AgNO3 5 K2CrO4 6 AgNO3 Net Ionic Equations: 4.