Editable DOCX - Andrew Shears
advertisement

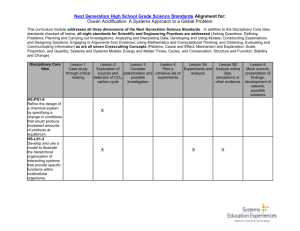
Lessons on Ocean Issues http://nobel48.geographer.me Ocean Acidification Prepared by Jonathan D. Steffen (student), Univ of Wisconsin-Fox Valley E-Mail: jonathansteffen1@gmail.com Web: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 2 Acknowledgements Prepared by Jonathan D. Steffen, an undergraduate student, as a capstone project for the GLG 291 seminar on ocean issues at the University of WisconsinFox Valley, under the direction of Dr. Beth A. Johnson and Dr. Andrew Shears. Funding for attendance at the Nobel 48: Our Global Issues conference at Gustavus Adolphus College in St. Peter, MN generously supported by funding from the Mielke Family Foundation of Appleton, Wisconsin. Funding for attendance at the 2013 Annual Meeting of the Association of American Geographers in Los Angeles, California, and the 2013 Posters in the Rotunda at the Wisconsin State Capitol in Madison, Wisconsin generously supported by funding from University of Wisconsin-Fox Valley Student Scholars. More information about the project, including editable lesson plans and additional resources, visit http://nobel48.geographer.me. This document and all of its components (unless otherwise cited to an outside source) are released to the Creative Commons. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 3 Figure 1.1: A healthy coral reef, with fish community (Flickr user ‘Sam and Ian’) About Ocean Acidification Anthropogenic emissions that are caused by the burning of fossil fuels, are releasing carbon dioxide into the atmosphere, in turn fundamentally altering chemistry of the global ocean waters. In short, ocean acidification is caused by increasing atmospheric concentrations of CO2, some of which dissolves into the ocean when it contacts the surface and forms a weak acid (carbonic acid) through a series of chemical reactions. Under increasing conditions of carbon dioxide exposure, additional acidity is produced in the oceans waters. This increase in acidity – alternatively referred to as the lowering of pH - threatens the bases of many food chains and other organisms that build their skeletons and shells out of calcium carbonate, which is a known buffering agent to acidic conditions. As ocean acidity continues to increase, the ability of marine ecosystems’ such as coral reefs and plankton communities to sustain themselves and continued calcification & growth greatly diminishes. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 4 In addition, many researchers have predicted that ocean acidification will have catastrophic consequences for these ecosystems within the next century. Nevertheless, ocean acidification remains one of the most misunderstood and under discussed set of environmental consequences that have ever resulted from human actions. Figure 1.2: Dead coral reef ecosystem (Flickr user ‘prilfish’) This lesson plan, with its extended learning experiences, will begin to familiarize students with the causes and effects of ocean acidification: the process by which our ocean is becoming increasingly acidic. By constructing this brief overview and lesson plan, it has become a goal to inform the generational youth about ocean acidification through simple hands-on experiments, informed instruction, and peer discussion. In each lesson, the students will build on their previous scientific education by continuing to apply proper scientific methods. The first hands-on lesson includes a simple experiment with minimal preparation, a short PowerPoint to aid the students through the procedure, and optional readings with worksheets. This experiment may take the course of few days to complete, really affording the student plenty of scientific observational time and opportunities for data collection. The instructor may also easily modify this lesson plan as needed to conduct further inquiry with additional forms of acid. Visit http://nobel48.geographer.me to download these resources. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 5 State Educational Standards This lesson meets the following states’ educational standards: Wisconsin Science Standards for 5-8 A.8.1 Develop their understanding of the science themes by using the themes to frame questions about science-related issues and problems A.8.6 Use models and explanations to predict actions and events in the natural world C.8.6 State what they have learned from investigations*, relating their inferences* to scientific knowledge and to data they have collected C.8.7 Explain* their data and conclusions in ways that allow an audience to understand the questions they selected for investigation* and the answers they have developed E.8.1 Using the science themes, explain and predict changes in major features of land, water, and atmospheric systems E.8.6 Describe through investigations the use of the earth's resources by humans in both past and current cultures, particularly how changes in the resources used for the past 100 years are the basis for efforts to conserve and recycle renewable and nonrenewable resources F.8.8 Show through investigations how organisms both depend on and contribute to the balance or imbalance of populations and/or ecosystems, which in turn contribute to the total system of life on the planet F.8.9 Explain how some of the changes on the earth are contributing to changes in the balance of life and affecting the survival or population growth of certain species F.8.10 Project how current trends in human resource use and population growth will influence the natural environment, and show how current policies affect those trends. Source: http://standards.dpi.wi.gov/stn_sciintro Illinois Middle/Junior High School 11.A.3a Formulate hypotheses testable by collecting data. 11.A.3b Scientific experiments control all but one variable. 11.A.3c Collect and record data accurately using consistent measuring and recording techniques and media. 11.A.3d Explain existence of unexpected results in a data set. 11.A.3f Interpret. represent results of analysis, produce findings. 11.A.3g Report and display the process and results of a scientific investigation. Source: http://www.isbe.net/ils/science/standards.htm Figure 1.3 (top): Healthy coral ecosystem (Flickr user prilfish). Figure 1.4 (bottom): Dead coral from same region (NOAA, public domain) Minnesota 8.2.1.2.1 Identify evidence of chemical changes, including color change, generation of a gas, solid 8.2.1.2.4 Recognize that acids are compounds whose properties include a sour taste, characteristic color changes with litmus and other acid/base indicators, and the tendency to react with bases to produce a salt and water. Source:http://education.state.mn.us/MDE/EdExc/StanCurri/K12AcademicStandards/index.htm In the second hands-on experiment, students will extend their experience by simulating the process of ocean acidification with their own breath. This also requires minimal preparation and may take approximately one hour to effectively complete. For this lesson, a PowerPoint that carries Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 6 students through the procedure and a handout is also available from http://nobel48.geographer.me. This lesson plan is based upon the educational standards set forth by the National Science Education Standards (1996) & the Board on Science Education (BOSE). It is also consistent with a number of regional standards and benchmarks including the States of: Wisconsin, Illinois, and Minnesota (see inset, page five). The purposes of this lesson plan will remain focused to accommodate students that are enrolled in scientific learning programs within grade levels 5-8. Skills: By grade levels 5-8, students should have been developing the abilities necessary for scientific inquiry, including concrete and formal thinking skills, such as problem solving and assessment. They should have a general understanding of the scientific process through their educational experience between grade levels K-4. The abilities that are necessary to carry out the scientific method, as well as a working knowledge of chemical properties and their changes, are typically developed between grade levels 5-8 according to acceptable content standards. The intent of this lesson plan is to continue to develop these skills within students currently enrolled in grade levels 5-8. It is then the purpose of this lesson plan to tie these scientific skills into the known cause and effect relationships of global ocean acidification. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 7 Impacts of Ocean Acidification Activity Objectives: Literacy skills through note taking and journaling. Students should be actively taking notes and or journals to describe their observations and measurements, as well as actively discussing the process and results of their experiments. Data collection and graphing skills by practice. Students should be collecting data relative to this experiment and synthesizing that information into a graph. The data collected could include: time to dissolve, weight of shells, before and after pH measurements. Students also should be doing preliminary research to discover what types of organisms are sensitive to acid and calcium, and be able to give examples. Connection to Students & Previous Lessons: Students continue to expand their knowledge of marine species and environments, and will establish introductory familiarity with various calcium-dependent marine species through facilitation of this lesson plan. Ocean acidification affects marine species differently. Some of the most obviously effected marine species will be more familiar to students at grade levels 5-8, including: coral reefs, plankton, and other shell producing ocean organisms that require calcium carbonate. A PowerPoint presentation has been assembled to guide instructors, and is available along with other course supplements: http://nobel48.geographer.me Materials (see Figure 2.1): A sufficient number of clear containers, preferably 50mL chemical lab glassware. Otherwise, these containers would need to be suitable for use with mild acids like vinegar. One per student or peer group is needed A sufficient amount of distilled white vinegar, 5% acidity. This is generally available at most grocery markets, and it should be procured with class size in consideration. An ideal amount of vinegar per student or peer group would be approximately four (4) ounces of vinegar. A sufficient amount of seashells, corals, limestone, eggshells, or other calcium/calcium carbonate based materials. Note that larger pieces of calcium material take longer to completely dissolve in any acid. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 8 A scientific scale capable of measuring increments of grams. Figure 2.1: Materials for this activity, including vinegar, chemical glassware, gram scale, sea shells. The use of seashells for this activity is highly recommended, as it relates more to previous ocean biology lessons the students should have already been exposed to. The educational outcomes will be more effective than the use of any other calcium based material due to the seashells corporeal connections to marine animal life. Seashells are generally available through most scientific supply companies; however if access to such outlets is limited, seashells are readily obtainable at a minimal cost at pet stores, or can be collected on a field trip to your local water habitats or shorelines. It’s important to note that if a field trip should occur, previous inquiry into the site rules and regulations on sample collection should be performed and respectfully observed. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 9 Engaging Demonstration for Instructor Figure 2.2: Coral in hydrochloric acid. (photo: Jonathan Steffen) If possible, the instructor may procure some hydrochloric acid with a concentration of 10% acid to 90% Water, and begin dissolving seashells or other calcium based materials by placing them into a small beaker of the acid (see Figure 2.2). Since hydrochloric acid is a stronger acid than vinegar, it is not recommended for student use even though the risks are only marginally greater. The stronger acid will cause the calcium to dissolve much more rapidly, and this dramatic reaction will engage students in preparation for their own vinegar-based experiments. A video of this demonstration is provided in the supplemental materials for this lesson: http://nobel48.geographer.me Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 10 First Hands-On Activity For this lesson plan, a simple experiment to visually depict the effects of ocean acidification on calcium dependent organisms has been tailored to fit within the aforementioned content standards. Expansion of the following experiment is easily accomplished by observing and documenting the effects of other forms of acidic liquids simultaneously or without the vinegar. For example, the students could use lemon juice, carbonated beverages or even some household cleaning products to compare results. Procedure: 1. Fill desired containers with 40mL of distilled white vinegar or other preferred acid (Figure 2.3). 2. Have the students weigh the calcium object (Figure 2.4) before placing the calcium materials into a vinegar filled container (Figure 2.5). 3. Ask the students to observe and document the reaction that occurs over time. Depending on how thick the calcium is and how fast it dissolves, the students can be asked to use a scale display their findings. The students could look for holes or other inconsistencies within the calcium and count how many they see over the course of a fixed timeframe and the plot that information on an (X,Y) graph. To take this part of the experiment further; if it’s possible while the calcium is dissolving, it will inflate full of gas. By doing this, the students will get a visual Figure 2.3 (top): 40mL of vinegar Figure 2.4 (middle): Weight before. Figure 2.5 (bottom) Dropping in vinegar Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 11 demonstration of the amount of carbon dioxide that is released as a result of acidification induced calcium reactions. A brief video demonstrating this activity is available with the supplemental materials for this lesson from http://nobel48.geographer.me 4. Following the completion of each set of measurements, a brief discussion can be encouraged among the students to build connections to the scientific ideas and foster scientific thought. 5. During the discussion, it may be important to consider the following key points, and encourage students to use research and reasoning skills to answer each question: Figure 2.6 (top): HCL vs Vinegar Figure 2.7 (bottom): Weight after. Why does the vinegar dissolve the shells? What gas is being released in this reaction? How does this affect ocean organisms that build shells? How does this affect the ocean food chains and stability? How would food chain stability affect coastal populations? Science Skills Used: The students will practice the steps of the Scientific Method, as well as gain experience in preparing and measuring materials for scientific experimentation. Also, by properly arranging their experiments themselves, students will gain confidence preparing for and conducting scientific experiments overall. The students’ scientifically orientated observational skills will become developed further over the course of an ongoing experiment. Try having the students compare and describe the different observable effects between the various acids that were used either hands on, in class, or for the Engaging Demo. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 12 Applied Math/Language Arts Skills: Instructors will build math skills within the students by requiring them to document and calculate the rates of calcium dissolution over time. This can be done by measuring the weight of shells at each interval of time during the course of the experiments. These kinds of measurements also could be done with varied levels of acidity by diluting the vinegar mixtures with purified water. The students could then begin plotting the information they gather on a graph. Language and social skills are also reinforced in the peer discussion phase of the experiment. Also, an instructor could require the student to complete a series of notes or journals that document the students’ observations and overall findings. Another hands-on activity on the next page! Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 13 Second Hands-On Activity To extend the classroom experience, another simple acidification experiment has been designed for the purposes of this lesson plan. The extended experience experiment demonstrates that carbon dioxide in breath can acidify small quantities of liquid in short periods of time. This experiment connects the anthropogenic emissions of carbon dioxide with the known culprit of ocean acidification, which is carbon dioxide dissolution into seawater. By making these acidification connections visual and through discussion, the student will better understand the causal relationship between carbon dioxide emissions and the changes in calcium dependent marine organisms. Additional Materials: 3mL of distilled water 1mL of a calcium (chloride) solution – Available from science and/or Pet stores as a commercial coral supplement or limestone slurry. Phenolphthalein base indicator solution Small beaker or flask Procedure: 1. Mix 3mL of distilled water with 1mL of the calcium solution in the beaker or flask. 2. Add 2-3 drops of phenolphthalein base indicator solution, or as needed until the solution turns pinkish-red. 3. Ask the students to blow or simply breathe across the mixture, sometimes agitating the mixture, until the solution returns to a clear liquid. Ask student to record approximate time of breathing required to “acidify” the liquid, and the approximate changes in color over the duration of the experiment. 4. Following the completion of each set of measurements, a brief discussion can be encouraged among the students to build connections to the scientific ideas and foster scientific thought. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me Lessons on Ocean Issues Ocean Acidification 14 Lesson Summary By completing this lesson, students should understand the impacts of ocean acidification on calcium-based sea life (from the instructor demonstration and first hands-on activity), as well as the anthropogenic causes of ocean acidification (as demonstrated in the second hands-on activity). Additional resources for this lesson, and other oceans lessons, are available for free download and use from http://nobel48.geographer.me Imagery Sources Figure 1.1: Flickr user ‘Sam and Ian.’ “Coral Reef.” 2008. http://www.flickr.com/photos/sam_and_ian/89250252/. Released by author to the Creative Commons. Figure 1.2: Flickr user ‘prilfish.’ “Dead corals.” 2010. http://www.flickr.com/photos/silkebaron/5122082306/. Released by author to the Creative Commons. Figure 1.3: Flickr user ‘prilfish.’ “Corals.” 2010. http://www.flickr.com/photos/silkebaron/5122082306/. Released by author to the Creative Commons. Figure 1.4: Burdick, David. Reef3206. N.d. NOAA's Coral Kingdom Collection. http://www.photolib.noaa.gov/htmls/reef3206.htm Public domain. Figures 2.1-2.7: Photographs by Jonathan D. Steffen. Released by author to the Creative Commons. Bubbling coral image in supplemental PowerPoint: Korallenriff Mit Lokal Stark Erhöhter CO 2 Konzentration. N.d.. http://www.mpibremen.de/Ozean-Versauerung_und_Korallenriffe.html, 5/31/2011. Video in supplemental clips: Filmed by Jonathan D. Steffen on the campus of the University of Wisconsin-Fox Valley. Prepared by Jonathan D. Steffen (jonathansteffen1@gmail.com), undergraduate at UW-Fox Valley. For digital copies and additional teaching resources on ocean issues: http://nobel48.geographer.me