Final-RGO-Paper
advertisement

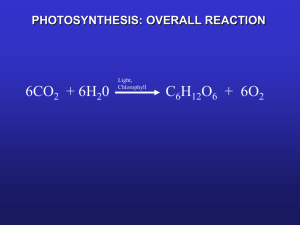
2013 Reduced Gravity Education Flight Program - Chlorella Growth Chamber Flight Date April 9, 2013 PRINCIPAL INVESTIGATORS Dr. Debora Wines; Life Science Instructor, Billings Central Catholic High School, Billings, MT CO-INVESTIGATORS Kylee Hraban; Billings Central Catholic High School James Dilts; Billings Central Catholic High School Nathan Heldt; Billings Central Catholic High School Laura Anne Westwood; Billings Central Catholic High School Introduction: The purpose of this study is to optimize growth conditions for algae in microgravity. Its importance for the International Space Station (ISS) is centered on carbon dioxide (CO2) remediation and oxygen production through photosynthesis, a biological process where sunlight, water and carbon dioxide are converted into sugar and oxygen. The experiment utilizes the green alga Chlorella pyrenoidosa as a model system for the study of photosynthesis and optimization of growth conditions in microgravity. Chlorella is a highly efficient photosynthetic organism with a simple nature and the highest amount of chlorophyll of any discovered organism, according to an article from Connecticut College (2004). The experiment attempted to grow algae embedded in solid media with greater or equal growth in comparison to a liquid media. Our solid media of choice was agar. Agar is a semi solid growth media that is viscous enough to be unaffected by changes in gravity. Agar is made by combining a hydrocolloid from seed weed extract with water using heat and pressure to create a gelatinous structure able to grow cultures. Typically cultures that are grown using agar are maintained on the surface of the agar, which results in a smaller volumetric growing area. In order to solve this problem, a method had to be developed to inoculate the algae inside the agar and allow it to grow. With this accomplished a feasible and cost effective life support system could be explored for the ISS that utilizes agar as its growth media. Abstract The experiment focused upon the creation of optimal algal growth conditions in a solid growth media. Optimizing growth conditions for the efficient growth of the alga Chlorella pyrenoidosa in microgravity would have several potential beneficial uses for the space environment. Short term benefits include the remediation of CO2 and production of oxygen via photosynthesis. Chlorella is especially valuable for this application because of its high chlorophyll content. For more long term spaceflight, Chlorella could be grown as a food and fuel source due to its high nutrition content and its production of combustible lipids. Chlorella cultures are normally maintained in liquid media. However, liquid cultures are not easily maintained in microgravity and would be subject to malfunction. The goal of this study was to optimize the growth of Chlorella in a solid agar growth media. Chlorella was embedded in small blocks of agar to provide a growing area comparable to that of a liquid culture, but easier to handle. Half of the samples contained agar infused with carbon dioxide. Molds for the agar blocks were sealable to minimize evaporation, but allowed for electronic sensors which measured carbon dioxide utilization and optical density as an indicator of photosynthesis efficiency. The experiment was run on the Zero Gravity flight at Ellington Field on April 9, 2013 for 64 parabolas. Chlorella grew in agar successfully in all samples, and grew the same rate in altered gravity as in normal gravity after an initial period of decreased growth. Throughout the study, algae growing in carbonated agar performed more efficiently than the non-carbonated sample. Results also showed that algae growing in agar performs 30% more efficiently in agar than in liquid media. These results suggest that agar is a suitable growth media in microgravity conditions. Growth of Chlorella in agar could enhance its practical uses in space, providing essential life support services at a reduced cost. Statement of the research problem: The use of algae has long been considered for the remediation of carbon dioxide and production of oxygen in space environments. Chlorella was one of the first organisms sent into space in the early 1960s by Herb Ward at Rice University. His results showed that algal growth was not affected by microgravity. Later experiments determined that around 50 liters of algae was required to support one person per day (Bovee et al., 1962). A problem with the use of algae is the strange behavior of liquids in microgravity. Most conventional growing methods involve pumps and aerators, which fail often in microgravity. This experiment uses a solid growing media to avoid these problems. Methods The research began with an examination of the International Space Station, which vents waste carbon dioxide gases into space. Next Chlorella was selected as the model system due to its high photosynthetic rates and potential benefits. The hypothesis was that carbonated agar would grow algae more efficiently than other variants and that altered gravity would not cause major change to the growth rates of the algae. Tests were conducted on the ground to determine the normal growth rates and the differences between media types, which showed that algae can successfully grow in agar with 30% more efficiency. Chlorella The Chlorella sample was obtained from Carolina science supplies. This pure sample was then cultivated, utilizing sterile technique, to provide a stock of Chlorella Pyrenoidosa to be used for experimentation. Agar Agar was obtained from Home Science Tools. One percent noncarbonated agar was made using 100 ml of spring water, 1 g of agar powder, 2 ml of algae nutrient concentrate (acquired from Carolina Science Supplies), which was then autoclaved for 20 minutes at 15 psi. Carbonated water was added to dilute the 2% agar before inoculating the algae when the agar is liquid at 55° C. Therefore, the agar was diluted to a 1% concentration. Using carbonated water could potentially change pH, but the nutrient concentrate contained the buffer TRIS which prevents the acidification of the agar by maintaining a pH of 6.8. Once the agar is prepared, it can be reheated into a liquid state and inoculated with algae. Chlorella was inoculated at 50°C using sterile technique and micropipettes. One milliliter of algae was added to 10 ml of agar for each sample for experimentation. Environmental monitoring Internal gravitational acceleration was measured by the use of an analog accelerometer. A reading of gravitational acceleration was taken every second and then stored on a micro-SD card utilizing a data logger. The readings were graphed post-flight in correlation with readings of optical density and carbon dioxide concentration (Figure 3). Optical Density Optical density is the measurement of how much light passes through a substance. As Chlorella grows, more and more cells are created through mitosis. As this occurs, less light penetrates the agar. Two photo resistors were used to measure the optical density of Chlorella. The photo resistors stored a reading, in bytes, of how much light was reaching the sensor on a micro-SD card. These readings were then graphed correlating with the accelerometer (Figure 3). Carbon Dioxide Remediation Carbon dioxide remediation was measured using an MG-811 sensor coupled with a SEN-0007 module obtained from Sandbox Electronics. By measuring the amount of carbon dioxide present the rate at which the Chlorella is photosynthesizing can be deduced (Figure 4). Data Logging An Open Log data logger was utilized to record data. The data logger was programmed to record digital readings from the sensors and store them in flash memory onto a 16 GB micro SD card. In total over 52,000 different data points were recorded over the two flights, each lasting 1.7 flight hours. Growth Chamber Design A B C Figure 1. Shows the schematics of our sample containers (picture A) our Lexan containment apparatus (picture B) and the apparatus fitting inside a NanoLab (picture C) In order to comply with safety standards set forth by the Reduced Gravity Flight Education Program (RGFEP), the Chlorella Growth Chamber (CGC) was designed to maximize growing area for Chlorella, allow adequate room for sensors, and fit into a NanoLab. The CGC was designed to fit in a 1.5U (10cm x 10cm x 15cm) NanoLab, made from 1/8” aluminum with 1/8” plastic covers for each side. Each plastic cover has 4 holes for bolts to secure the NanoLab to an International Space Station experiment rack. The NanoLab plastic lids have an area designed to fit USB cables in or through. The 1/8” aluminum protects users from electrical shock, electrical fires, or any sharp edges contained inside. A 5-sided rectangular prism made from 1/8” Lexan was designed to fit inside the NanoLab and house the electronics and samples. Each side of the Lexan apparatus is 1/16” smaller than the NanoLab allowing for the addition of padding in order to hold the Lexan box snugly inside. Lexan was chosen to house the CGC because of the cost, strength of material, non-frangibility, and it allows for visual troubleshooting. A notch was cut into one side of the CGC to allow the wires from the carbon dioxide sensors, light bank, and Arduino board to exit through the NanoLab’s plastic covers.(Figure 1: B and C) Four 1” cubed 1/8” Lexan boxes (Figure 1: A) were designed to allow for different types of agar inoculated with Chlorella to be tested: two carbonated and two noncarbonated. Each cube was designed so one cube could sit on top of the other and side by side when inside the CGC, maximizing spatial usage. One hole for a screw was designed into each side of the lid and the cube to keep the lid securely in place while one large hole was placed on the top of each lid to allow gas exchange to take place along with sensor access. A 1/16” rubber gasket was placed along the lining of the bottom of the inside of the lid to decrease water loss. Results CO2 concentration (PPM) The results from growth of Chlorella on the ground are presented in Figure 2. The more rapid consumption of carbon dioxide by Chlorella in agar indicated that more photosynthesis had taken place in comparison to liquid media. According to results (Figure 2), Chlorella in non-carbonated agar performs approximately 30% more efficiently than in liquid growth media. This proves our hypothesis that algal growth is successful in agar. 2000 1500 1000 Agar Figure 2. Carbon dioxide remediation rates of noncarbonated agar vs. a liquid media (data provided by A.M. A.D. and M.S. from the AP Biology class). Liquid Media 500 0 1 2 3 4 5 6 Time in Hours 7 8 Figure 3 is a graph of optical density. Optical density was used to quantify the biomass production (growth) of Chlorella in this study. It is a simple concept that uses a light intensity sensor positioned below the algae sample and the lights. As the algae grow inside the agar, less light will reach the sensor. The results are from the two flights on April 9th of how Chlorella grew while in parabolic flight. It is possible to see the decrease in the amount of light over the course of the two flights. Our results from the first flight show that the Chlorella experienced stress as seen by a decreased growth followed by periods of rapid growth. During the second flight the Chlorella appeared to have acclimated to the changes in gravity and grew at a normal rate, proving our second hypothesis. Changes in gravity were recorded by an accelerometer oscillations indicate parabolas. 140 Sensor Readings (bytes) 120 100 Break between flights. 80 60 40 Towards the end of the first flight the Chlorella began to acclimate. Gravity Non-Carbonated 20 Carbonated 0 Carbon Dioxide Changes Flight 2 Flight 1 Non-Carbonated Solid Ground 21 Carbonated Ground 1 0 0.1 0.2 Volts Figure 4. Changes in carbon dioxide levels between carbonated and non-carbonated agars on the ground and aboard the Zero-G plane. 0.3 The results from the CO2 sensors show that the Chlorella performed photosynthesis faster in an altered gravity environment. These results were taken when algae had been embedded in agar approximately five days. However, the CO2 sensor measuring the non-carbonated agar malfunctioned and returned no appreciable flight results. Carbonated agar performed better with a 16.6% increase in carbon dioxide consumption. During the first flight, Chlorella showed signs of stress which can be seen in the erratic growth patterns. During the second flight, the Chlorella samples actually became accustomed to the altered gravity environment and grew at the steady rate seen in preliminary ground experiments. These results suggest that Chlorella appears to easily acclimate to differences in gravity and would successfully grow in extended microgravity conditions such as on the ISS. The results also confirm that carbonated agar is more efficient at growing algae. Conclusion and Discussion Agar can be used as a solid media for algal growth, allowing for the circumvention of problems due to the enhanced cohesion of liquids in microgravity. According to our results Chlorella is more productive when embedded in agar in comparison to Chlorella grown in liquid media. Furthermore, Chlorella embedded in carbonated agar has a greater productivity in relation to non-carbonated agar. These results suggest that agar could be used with a lesser volume compared to liquid media in order to provide enough oxygen for a feasible life support system on the International Space Station. For further experimentation we will be evaluating the effect of surface area to volume in order to access whether the shape and dimensions of the containers could be changed to optimize growth and gas exchange. Also the consistency of the agar will be tweaked to promote easier growth and greater stability. This experiment will later be tested in extended microgravity on board the ISS. This experiment is important to future spaceflight for a variety of reasons. Most importantly growth in a solid media is important because of the strange behaviors of liquids in microgravity. Using solid media problems such as pump failure or leakage are bypassed. The main benefit for the use of algae on the ISS is that the astronauts will have peace of mind knowing that their air supply is not fully dependent on oxygen shipments, but is recycled onboard the ISS utilizing the natural process of photosynthesis. Currently, oxygen is shipped to the ISS at a cost of at least $10,000 per pound. Also, carbon dioxide is vented off of the ISS, because there is no current way to make use of it. Furthermore, if a shipment of oxygen were to fall behind or never arrive at the ISS, the astronauts would have no back-up system. Chlorella performs photosynthesis, which takes the carbon dioxide that the astronauts breathe out and converts it to sugar for the algae to use. The by-product of the reaction is oxygen. Approximately 50 liters of algae in a liquid media is required to produce enough oxygen for a human to breathe. Since the design makes use of agar instead of a liquid, the volume required to sustain a human is reduced because the concentration per cubic millimeter is higher. So if algae, such as Chlorella, could be effectively used for bioremediation of the air supply on the ISS this would not only reduce but could even eliminate the need for oxygen shipments. A further important benefit to the consumption of Chlorella is its radiation protection qualities. Chlorella extracts have been proven in Taiwan to protect skin fibroblasts from intense UVB; the Chlorella extract prevented enough damage to the DNA to keep the cell healthy (Shih, Mei Fen, and Jong Yub Cherng, 2012). For long term space exploration the algae could potentially be used as a food source that could supplement the diets of astronauts in long term space travel. Chlorella contains many vital nutrients for human sustenance. Algae are also much simpler to grow and take up less space than conventional crops for food. Although not all nutrients necessary for human functionality are found in Chlorella, it could serve as a large part of their diet if made palatable. Chlorella contains 58.4% protein, more than most meats. It also contains many vitamins necessary for good health, and 221 milligrams of calcium per 100 grams that will protect against bone loss. Algal biofuels have also been examined as a theoretical energy source. Outreach studying the effects of microgravity on the human body was conducted. Several items including a model USS Enterprise spaceship, a slinky, and a wind up toy were taken onboard the Zero-G plane to demonstrate microgravity conditions. Bibliography Becker, E.W. (2007). "Micro-algae as a source of protein". Biotechnology Advances 25 (2): 207– 10. doi:10.1016/j.biotechadv.2006.11.002. PMID 17196357. Bovee, H H; Pilgrim, A J; Sun, L S; Schubert, J E; Eng, T L; Benishek, B J. (1962). "Large Algal Systems" (PDF). Contrails. Illinois Institute of Technology. p. 12. Ward C.H., Wilks S.S., and Craft H.L. (1970). “Photosynthesis in Space” Dev. Indust. Microbiol.11:276295 "Chlorella." Chlorella. Connecticut College, 2004. Web. http://silicasecchidisk.conncoll.edu/LucidKeys/Carolina_Key/html/Chlorella_Main.html Shih, Mei Fen, and Jong Yub Cherng.(2012). "Protective Effects of Chlorella-Derived Peptide Against UVC-Induced Cytotoxicity through Inhibition of Caspase-3 Activity and Reduction of the Expression of Phosphorylated FADD and Cleaved PARP-1 in Skin Fibroblasts." Molecules. Chia-Nan University of Pharmacy and Science Acknowledgements Dr. Herb Ward, Rice University Dr. Florence Gold, NASA Research Integration Office, John Space Center Dr. Michael Johnson, NanoRacks Dr. Michael Roberts, Kennedy Space Center Dr. Andy Wildenberg, Rocky Mountain College Dr. Stan Wiatr, Montana State University Billings Energy Laboratories, Billings, Montana Dr. Dave Butler, Montana State University Billings BCCHS School Board and Principal Sheldon Hanser Exxon Mobil, Billings, Montana Contact Information Debora Wines, Ph.D., Instructor of Life Sciences Billings Central Catholic High School 3 Broadwater Ave Billings, 59101 406.861.0728 dwines@billingscatholicschools.org