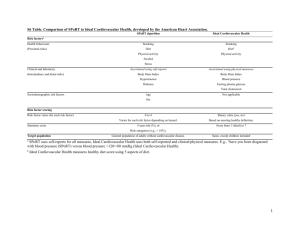
Review of cardiovascular safety of
non-steroidal anti-inflammatory
drugs and Safety review of
diclofenac
Summary and issues document
Version 1.0, October 2014
Therapeutic Goods Administration
About the Therapeutic Goods Administration
(TGA)
The Therapeutic Goods Administration (TGA) is part of the Australian Government
Department of Health, and is responsible for regulating medicines and medical devices.
The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management
approach designed to ensure therapeutic goods supplied in Australia meet acceptable
standards of quality, safety and efficacy (performance), when necessary.
The work of the TGA is based on applying scientific and clinical expertise to decisionmaking, to ensure that the benefits to consumers outweigh any risks associated with the use
of medicines and medical devices.
The TGA relies on the public, healthcare professionals and industry to report problems with
medicines or medical devices. TGA investigates reports received by it to determine any
necessary regulatory action.
To report a problem with a medicine or medical device, please see the information on the
TGA website <http://www.tga.gov.au>.
Copyright
© Commonwealth of Australia 2014
This work is copyright. You may reproduce the whole or part of this work in unaltered form for your own personal use or, if
you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the
reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that
reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other
rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or
otherwise) without first being given specific written permission from the Commonwealth to do so. Requests and inquiries
concerning reproduction and rights are to be sent to the TGA Copyright Officer, Therapeutic Goods Administration, PO Box
100, Woden ACT 2606 or emailed to <tga.copyright@tga.gov.au>
Confidentiality
All submissions received will be placed on the TGA’s Internet site, unless marked confidential. Any confidential material
contained within your submission should be provided under a separate cover and clearly marked “IN CONFIDENCE”.
Reasons for a claim to confidentiality must be included in the space provided on the TGA submission coversheet. For
submission made by individuals, all personal details, other than your name, will be removed from your submission before it
is published on the TGA’s Internet site. In addition, a list of parties making submissions will be published. If you do not wish
to be identified with your submission you must specifically request this in the space provided on the submission coversheet.
Therapeutic Goods Administration
Version history
Version
Description of change
Author
Effective date
V1.0
Original publication
Office of Product Review
07/10/2014
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 3 of 13
Therapeutic Goods Administration
Contents
1. Background _________________________________ 5
2. Cardiovascular risk review _____________________ 5
2.1 The review __________________________________________________________________ 5
2.2 Request for advice from the Advisory Committee on the Safety of
Medicines _______________________________________________________________________ 7
2.3 Consideration of report by innovator sponsors ______________________ 8
3. Safety review of diclofenac ____________________ 8
3.1 The review __________________________________________________________________ 8
3.2 Request for advice from the Advisory Committee on the Safety of
Medicines _______________________________________________________________________ 9
3.3 Consideration of report by innovator sponsors ____________________ 10
4. Conclusions ________________________________ 10
5. Recommendations __________________________ 11
Option 1 – Status quo ________________________________________________________ 11
Option 2 – Non-regulatory __________________________________________________ 11
Option 3 – Regulatory _______________________________________________________ 11
Label changes ------------------------------------------------------------------------------- 11
Re-scheduling OTC NSAIDs -------------------------------------------------------------- 11
Option 4 – Combination of regulatory and non-regulatory activities 12
6. Next steps _________________________________ 12
Making submissions _________________________________________________________ 12
Content of submissions ------------------------------------------------------------------ 12
What will happen? ------------------------------------------------------------------------- 12
Enquiries ------------------------------------------------------------------------------------- 12
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 4 of 13
Therapeutic Goods Administration
1. Background
The eight non-steroidal anti-inflammatory drugs (NSAIDs) celecoxib, etoricoxib,
indomethacin, meloxicam, piroxicam, diclofenac, ibuprofen and naproxen are available in
Australia as prescription medicines. Three of these NSAIDs, diclofenac, naproxen and
ibuprofen, are also available in lower dose forms without a prescription as either pharmacistonly or pharmacy only medicines. Low-dose oral ibuprofen and topical piroxicam are
unscheduled, available not only in pharmacies but also in supermarkets and other retail outlets,
and are widely used as analgesics. Pharmacist-only, pharmacy only and unscheduled medicines
are collectively known as over-the-counter (OTC) medicines.
The use of NSAIDs at prescription-only dosages is known to increase the risk of hypertension,
heart failure, myocardial infarction and stroke. Following a TGA review of the safety of these
medicines in 2005-2006, their Australian Product Information (PI) documents are now required
to include appropriate warnings regarding these risks under the heading ‘Precautions’. These
cardiovascular risks are also reflected in the Consumer Medicine Information (CMI) documents.
Diclofenac is also known to carry a risk of hepatotoxicity (liver damage).
There are several mandatory warnings and other advisory statements that must be included on
the labels of OTC products containing diclofenac, ibuprofen or naproxen. All products must
include the following warning statements relating to duration of use and concomitant use of
multiple pain relievers:
“Do not use for more than a few days at a time unless a doctor has told you to. Do not
exceed the recommended dose. Excessive use can be harmful”, and
“Unless a doctor has told you to, do not use this product with other products containing
[diclofenac/ibuprofen/naproxen], aspirin or other anti-inflammatory medicines or other
medicines you are taking regularly.”
However, the required statements currently do not warn of the risk of cardiovascular events and
OTC diclofenac products are not currently required to carry a warning statement regarding the
risk of liver damage.
To address this, the TGA undertook two reviews - a Review of cardiovascular safety of nonsteroidal anti-inflammatory drugs and a Safety review of diclofenac. This summary document
provides an overview of the conclusions from these reviews and provides four alternative
options to address the risks identified in the reviews. The options put forward focus specifically
on strategies to mitigate the risks associated with OTC NSAIDs.
Copies of the Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac can be found on the TGA website.
2. Cardiovascular risk review
2.1 The review
In early 2012, the TGA commenced an update to its 2005-2006 safety review of NSAIDs. A
search of the medical literature published from 2005 onwards was carried out and
approximately 200 papers that appeared relevant to the review were identified.
The 49 Australian sponsors of these medicines were provided with a list of the literature
references and invited to provide comment and any additional information that might be of
relevance to the Review. Submissions were received from the following:
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 5 of 13
Therapeutic Goods Administration
1.
Australian Self-Medication Industry Inc (ASMI)
2.
Abbott Australasia Pty Ltd
3.
Alphapharm Pty Ltd
4.
Boehringer Ingelheim Pty Ltd
5.
Novartis Consumer Health Australasia Pty Ltd
6.
Novartis Pharmaceuticals Australia Pty Ltd
7.
Pfizer Australia Pty Ltd
8.
Reckitt Benckiser Pty Ltd
A review of the literature identified through the TGA’s search, other relevant information (such
as PI and CMI documents and adverse event reports), and the material submitted by the
companies and organisations listed above was undertaken. The reviewers:
1.
carried out a literature-based review of the cardiovascular safety of diclofenac, naproxen
and ibuprofen and provide a comparative risk-benefit analysis of these medicines when
used at the dosages available with and without prescription
2.
compared the cardiovascular safety of diclofenac, naproxen and ibuprofen with that of the
five NSAIDs available only on prescription
3.
reviewed the current warnings in the PI and CMI documents for all eight NSAIDs and
provide advice on the appropriateness, consistency and adequacy of these warnings and
any changes that might need to be made
4.
provided advice on any changes that might need to be made to the warnings on the labels
for OTC products containing diclofenac, naproxen and ibuprofen.
The review included a number of recommendations for changes to the PI documents and labels
of the different medicines reviewed. Specifically that:
1.
all eight NSAIDs reviewed are associated with significantly increased risks of stroke, heart
attack or other cardiovascular events and they should be used with caution in patients with
predisposing cardiovascular risk factors
2.
there is a need for more awareness amongst health professionals and patients regarding the
cardiovascular risks associated with all NSAIDs
3.
the cardiovascular risks and necessary precautions are described in the current PIs, but
there is a need for greater consistency in the wording used and for prescribers to be made
more aware of the importance of assessment of risks in each individual patient, the
increased risk of cardiovascular events (especially in patients with prior cardiovascular
disease or cardiovascular risk factors) and the need for periodic assessment to detect any
signs or symptoms indicating cardiovascular events associated with NSAID treatment
4.
no major changes to the availability of OTC NSAIDs (diclofenac, ibuprofen and naproxen)
are needed. These medicines provide effective pain relief when used according to the label
at recommended doses for short durations
5.
the risk of gastrointestinal bleeding is well covered in the labels for OTC products
6.
there is a need to increase consumer awareness about the cardiovascular risks associated
with these medicines through additional and stronger label warnings about those risks via
amendments to the Required Advisory Statements for Medicine Labels (RASML). The
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 6 of 13
Therapeutic Goods Administration
addition of the following information and warnings to the labels of OTC NSAIDs would help
to ensure safer use.
a.
‘NSAIDs may cause an increased risk of serious cardiovascular thrombotic events,
myocardial infarction, and stroke, which can be fatal. This risk may increase with
duration of use. Patients with cardiovascular disease or risk factors for cardiovascular
disease may be at greater risk.’ [or words to that effect]
b.
Stronger reminders that patients with cardiovascular disease and/or cardiovascular
risk factors should seek the advice of a health professional before using these NSAIDs.
Health professionals and patients should remain alert for cardiovascular events even in
absence of previous cardiovascular symptoms. Patients should be informed about signs
and/ or symptoms of serious cardiovascular toxicity and the steps to take if they occur.
c.
Stronger reminders about limiting the dose and duration of treatment in accordance
with the package instructions unless otherwise advised by a health professional.
2.2 Request for advice from the Advisory Committee on the
Safety of Medicines
Advice on the review was sought from the Advisory Committee on the Safety of Medicines
(ACSOM) at its meeting on 9 November 2012. The Committee was asked to provide advice on
whether the changes to the PI, CMI and product labelling would be sufficient to mitigate the
cardiovascular risks identified in the review.
ACSOM advised that while changes to the PI, CMI and product labelling would be important,
labelling changes alone were insufficient to mitigate the risk of adverse cardiovascular events
associated with the use of the OTC NSAIDs ibuprofen, naproxen and diclofenac.
The Committee advised that taken in totality, the evidence regarding cardiovascular risks
associated with NSAIDs was sufficiently compelling that the TGA should give consideration to
other measures to mitigate the risks associated with the use of NSAIDs. Given the safety
concerns, the committee supported the need for additional risk mitigation activities to limit OTC
NSAID use and raise awareness of the risks associated with the use of these medicines.
With regard to any proposed label changes, ACSOM advised that that any warnings on the labels
need to be clear and be understood by consumers who will not be consulting a health
professional prior to purchasing these medicines OTC.
ACSOM was particularly concerned that the cardiovascular safety profile of diclofenac appears
to be similar to that of other cyclooxygenase-2 (COX-2) inhibitors1 which are only available on
prescription. This, coupled with the additional risks of hepatotoxicity, warrants further
consideration. ACSOM advised that the TGA give consideration to undertaking a full risk-benefit
review of diclofenac.
There are two major classes of NSAIDs, the traditional NSAIDs and the selective COX-2 inhibitors. It is
well known that the traditional NSAIDs are associated with serious gastrointestinal side effects. The COX2 inhibitors were developed to reduce the gastrointestinal side effect profile while maintaining the
efficacy of the traditional NSAIDs. COX-2 inhibitors are known to be associated with high rates of
cardiovascular adverse events. The first COX-2 inhibitor (celecoxib) was registered in Australia in 1999.
1
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 7 of 13
Therapeutic Goods Administration
2.3 Consideration of report by innovator sponsors
In May 2014, following redaction of information identified as commercially confidential, a copy
of the Review of cardiovascular safety of NSAIDs was provided to the sponsors responsible for
the innovator products for their comment prior to wider consultation with other stakeholders.
Several of the sponsors provided comment and, following consideration of those comments,
small editorial amendments were made to the review to improve accuracy and clarity. The
conclusions and recommendations in the review have not been affected by those amendments.
3. Safety review of diclofenac
3.1 The review
Following consideration of the advice from ACSOM that the TGA should give consideration to
undertaking a further review of diclofenac, a literature-based review of the safety of diclofenac
was commenced. The review included the following conclusions and recommendations.
1.
There is no emerging safety information regarding gastrointestinal or hepatic effects. The
risk/benefit remains favourable and the PI conveys appropriate warnings/precautions.
There is consistent evidence that there is an increased risk of serious cardiovascular events
with the use of diclofenac. The overall risk/benefit remains favourable however the PI
needs to updated to more appropriately convey the risks. It is recommended that:
–
post coronary artery bypass graft pain relief and treatment of severe heart failure be
added to the list of contraindications.
–
stronger warnings about the cardiovascular risk be added to the precautions together
with the need to carefully consider the risks and benefits of treatment in individuals at
higher risk of cardiovascular disease.
2.
The use of diclofenac as an OTC product for short term use at a low dose remains
appropriate. The current RASML for OTC products contain sufficient warnings regarding the
potential for gastrointestinal side effects. However they do not contain adequate warnings
about the potential risk of cardiovascular side effects or serious hepatic side effects. The
RASML statement ‘do not use if you have heart failure’ does not adequately cover the risk to
those with heart disease who have not developed heart failure and those at high risk of
heart disease. The addition of warnings to convey the risk to those people with heart
disease and the risk of serious hepatic side effects is warranted.
3.
Based on the available information the risk/benefit profile for topical diclofenac remains
favourable. There is a paucity of evidence of serious systemic side effects with topical
diclofenac. Despite this relative lack of evidence it is recommended that the CMI or package
insert (as appropriate) for topical diclofenac include warnings that systemic absorption is
likely and that adverse cardiovascular events have been associated with oral diclofenac.
4.
All of the above changes should be communicated via a TGA web statement, a Medicines
Safety Update article and liaison with NPS MedicineWise.
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 8 of 13
Therapeutic Goods Administration
3.2 Request for advice from the Advisory Committee on the
Safety of Medicines
Advice was sought from ACSOM on the Safety review of diclofenac at its meeting on 7 March
2014. Specifically, the Committee was asked to provide advice on whether on whether the
inclusion of additional warnings on the labels of diclofenac-containing medicines regarding the
potential risk of cardiovascular and liver side effects was an appropriate risk minimisation
activity, whether ACSOM considered any additional risk minimisation activities should be
implemented and the strength of the evidence to support the safety of topical diclofenac
products.
The Committee supported the conclusions and recommendations in the review regarding the
cardiovascular and hepatotoxicity risks associated with diclofenac. ACSOM agreed that COX-2
inhibitors have a well-established increased risk of adverse cardiovascular events. The
committee also agreed that the elevated risk of cardiovascular events with diclofenac was more
comparable to the highly COX-2 selective agents, which are prescription-only medicines.
The committee discussed the results from observational studies which showed that the
increased cardiovascular risk for diclofenac was not limited to patients with a pre-existing
history of heart disease. Patients at risk of heart disease, such as patients with vascular disease
risk factors or diabetes, also had an increased risk of cardiovascular events with the use of
diclofenac. ACSOM noted that a large proportion of the population has a low to moderate risk of
heart disease and as many people at risk of heart disease are undiagnosed or are unaware that
they have risk factors, the cardiovascular risks associated with oral diclofenac should be
communicated widely to encompass these individuals.
ACSOM advised that the inclusion of additional RASML statements on OTC oral diclofenac
products would be an appropriate risk minimisation measure. The committee advised that new
statements should convey that the risk applies more broadly than just for those with preexisting heart disease, and that those at risk of heart disease are also at increased risk.
ACSOM considered that there was a lack of awareness in the general community as to the
cardiovascular risk of OTC NSAIDs and in particular, diclofenac. ACSOM advised that the use of
publications such as those from NPS MedicineWise and the Medicines Safety Update may
increase awareness and correct undue perceptions of safety for these products among health
professionals.
With regard to adverse effects on the liver, ACSOM noted that there were no genetic risk factors
for liver injury. The committee discussed the putative mechanism by which diclofenac causes
adverse liver effects. ACSOM noted that an increased risk of a rise in liver enzymes was observed
with diclofenac in comparison with most NSAIDs, however the absolute risk of serious adverse
events was low. ACSOM considered that, as diclofenac can be purchased OTC, there is the
potential for no, or only limited, monitoring of patients using these products and therefore early
signs of hepatotoxicity may be missed.
ACSOM noted that there was a perception that the level of access to the consumer is a direct
measure of safety and that the availability of oral diclofenac as an OTC medicine may give a false
perception of safety to the general public.
With regard to the safety of topical diclofenac products, ACSOM agreed that there was a lack of
evidence related to the risk of topical diclofenac products and therefore no firm conclusions
could be drawn. The safety of topical diclofenac was largely derived from the assumption that
bioavailability of topical diclofenac products is low in comparison to oral diclofenac products
and that there is a lack of adverse events associated with topical diclofenac.
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 9 of 13
Therapeutic Goods Administration
The committee discussed that the bioavailability of topical diclofenac products was dependent
on the formulation of the product. Therefore ACSOM agreed that there was a small residual risk
of hepatotoxicity and adverse cardiovascular and gastrointestinal events with topical diclofenac,
particularly if used in excess. However, based on the evidence available, ACSOM advised that the
safety profile of topical diclofenac appeared to be similar to that of traditional oral NSAIDs,
rather than the COX-2 selective agents.
ACSOM noted that there may be an unfounded perception among consumers that the localised
application of topical diclofenac did not result in systemic distribution of the drug. ACSOM
advised that it would be appropriate for the CMI or package insert (as appropriate) for topical
diclofenac to include warnings that systemic absorption is likely and that adverse cardiovascular
events have been associated with oral diclofenac. While the dosage of topical diclofenac is 10%
of the dose of oral OTC diclofenac, safety is based on an assumed short term exposure and low
bioavailability and therefore a theoretical risk exists with sustained exposure. However, ACSOM
advised that that this theoretical risk should not be overplayed, as the exposure metrics of
topical diclofenac are different to those for oral diclofenac, particularly high dose oral diclofenac
and there is no evidence of a safety signal with topical diclofenac.
3.3 Consideration of report by innovator sponsors
In May 2014 a copy of the Safety review of diclofenac was provided to the sponsors responsible
for the innovator prescription and OTC products containing diclofenac for their comment prior
to wider consultation with other stakeholders. The sponsors provided comment and, following
consideration of those comments, small editorial amendments were made to the review to
improve accuracy and clarity. The review’s conclusions and recommendations have not been
significantly affected by those amendments.
4. Conclusions
The TGA’s overall conclusions based on these two reviews of the risks associated with the use
the NSAIDs available in Australia are as follows.
All eight NSAIDs celecoxib, etoricoxib, indomethacin, meloxicam, piroxicam, diclofenac,
ibuprofen and naproxen are associated with significantly increased risks of stroke, heart
attack or other cardiovascular events and they should be used with caution in patients with
predisposing cardiovascular risk factors.
There is a need for more awareness amongst health professionals and patients regarding
the cardiovascular and hepatotoxicity risks associated with NSAIDs. While these risks and
necessary precautions are described in the current PIs, there is, in some cases, a need for
greater consistency in the wording used and for prescribers to be made more aware of the
importance of assessment of risks in each individual patient, the increased risk of
cardiovascular events (especially in patients with prior cardiovascular disease or risk
factors) and the need for periodic assessment to detect any signs or symptoms indicating
cardiovascular events associated with NSAID treatment.
These medicines provide effective pain relief when used according to the label, at
recommended doses and for short durations of time. However, there is a need to increase
consumer awareness through additional and stronger label warnings about the
cardiovascular risks associated with these medicines, and the need for patients with
cardiovascular disease or risk factors to consult a health professional before using them.
Consumers also need to be made more aware of the need to limit the dose and duration of
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 10 of 13
Therapeutic Goods Administration
treatment in accordance with the package instructions unless otherwise advised by a health
professional.
The Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and the Safety
review of diclofenac can be found on the TGA website.
5. Recommendations
There are four alternative options being put forward in this consultation document. The options
put forward focus specifically on strategies to mitigate the risks associated with OTC NSAIDs.
Any action undertaken is designed to increase awareness amongst consumers and health
professionals of the risks associated with NSAID use and therefore decrease the number of
adverse events. In this context, option 1 is not considered to be a viable option.
The four proposed options are:
Option 1 – Status quo
No changes are made to labels; nor are any communication activities undertaken to raise health
professional and consumer awareness.
Option 2 – Non-regulatory
No changes are made to labelling and no changes are made to the scheduling. Instead TGA uses
existing communications channels and publications and also works with stakeholders such as
NPS MedicineWise to raise awareness and educate health professionals and consumers on the
risks associated with the use of NSAIDs.
Option 3 – Regulatory
Label changes
TGA requires sponsors of pharmacist-only, pharmacy-only and unscheduled OTC NSAIDs to
update their labelling information (and/or CMI, as applicable) or are required to have a CMI
supplied with the product as a package insert.
The labelling changes could be imposed through:
an updated to RASML with the additional required advisory statements
a change of condition of registration under Section 28 of the Act.
Re-scheduling OTC NSAIDs
All unscheduled and pharmacy-only OTC NSAIDs could be considered for re-scheduling to be
pharmacist-only to ensure the intervention of pharmacist at the time of supply. If rescheduled,
these products would still require changes to their labelling.
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 11 of 13
Therapeutic Goods Administration
Option 4 – Combination of regulatory and non-regulatory
activities
TGA requires sponsors of pharmacist-only, pharmacy-only and unscheduled OTC NSAIDs to
update their labelling information as outlined in Option 3. Labelling updates would be
complemented by communications activities undertaken by the TGA in collaboration with
existing stakeholders.
6. Next steps
Making submissions
Content of submissions
Submissions may address any, or all, of the options list above.
In addition, submissions might include:
suggested improvements or alternatives to the options.
whether or not you support the specific or parts of options or a combination of options. If
you do not support the options you may make suggestions for an alternative that is
acceptable to you.
an assessment of how the proposed options will impact on you. That is, what do you see as
the likely benefits or costs to you (these may be financial or non-financial). If possible,
please attempt to quantify the direct and indirect costs and benefits.
your views in relation to other benefits and costs that you consider relevant.
Any submissions will inform the final decision which will be taken by the TGA.
What will happen?
Submissions will be reviewed and considered by the TGA and any updates on proposed actions
will be made available on the TGA’s Internet site.
Enquiries
Questions relating to the submission should be directed to the Head, Office of Product Review,
Dr Jane Cook by email to si.coordinator@tga.gov.au or by telephone to 02 6232 8188.
Consultation: Review of cardiovascular safety of non-steroidal anti-inflammatory drugs and
Safety review of diclofenac - Summary and issues document
V1.0 October 2014
Page 12 of 13
Therapeutic Goods Administration
PO Box 100 Woden ACT 2606 Australia
Email: info@tga.gov.au Phone: 1800 020 653 Fax: 02 6232 8605
http://www.tga.gov.au
R14/981826