Correct table of shapes
advertisement
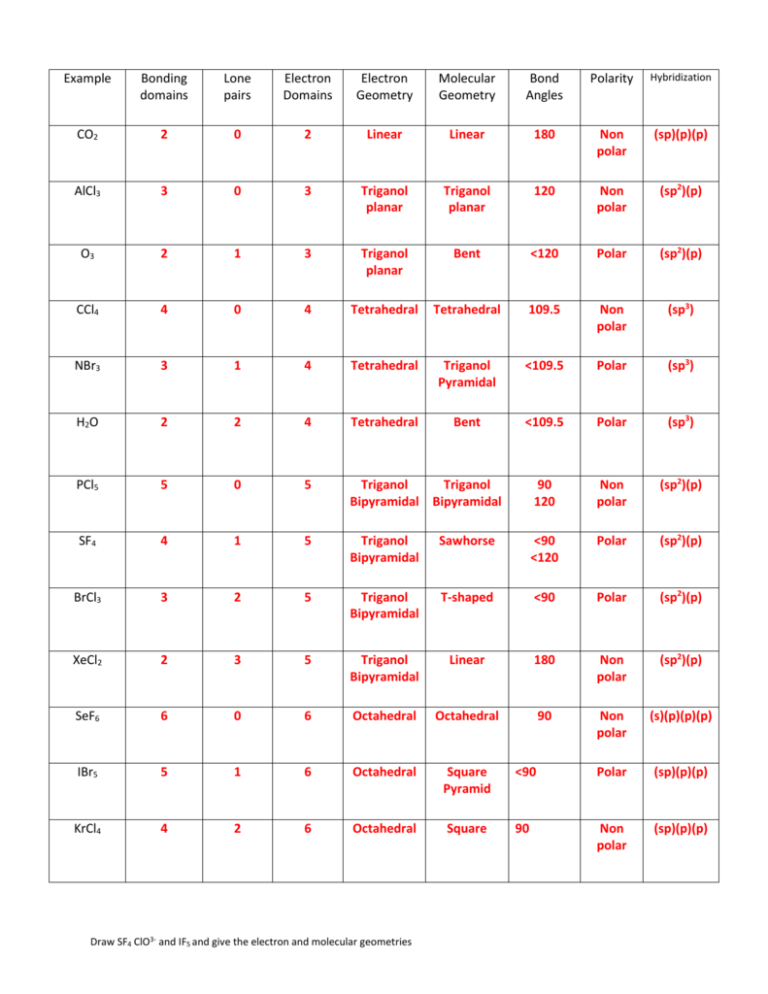
Example Bonding domains Lone pairs Electron Domains Electron Geometry Molecular Geometry Bond Angles Polarity Hybridization CO2 2 0 2 Linear Linear 180 Non polar (sp)(p)(p) AlCl3 3 0 3 Triganol planar Triganol planar 120 Non polar (sp2)(p) O3 2 1 3 Triganol planar Bent <120 Polar (sp2)(p) CCl4 4 0 4 Tetrahedral Tetrahedral 109.5 Non polar (sp3) NBr3 3 1 4 Tetrahedral Triganol Pyramidal <109.5 Polar (sp3) H2O 2 2 4 Tetrahedral Bent <109.5 Polar (sp3) PCl5 5 0 5 Triganol Triganol Bipyramidal Bipyramidal 90 120 Non polar (sp2)(p) SF4 4 1 5 Triganol Bipyramidal Sawhorse <90 <120 Polar (sp2)(p) BrCl3 3 2 5 Triganol Bipyramidal T-shaped <90 Polar (sp2)(p) XeCl2 2 3 5 Triganol Bipyramidal Linear 180 Non polar (sp2)(p) SeF6 6 0 6 Octahedral Octahedral 90 Non polar (s)(p)(p)(p) IBr5 5 1 6 Octahedral Square Pyramid <90 Polar (sp)(p)(p) KrCl4 4 2 6 Octahedral Square 90 Non polar (sp)(p)(p) Draw SF4 ClO3- and IF5 and give the electron and molecular geometries Draw SF4 ClO3- and IF5 and give the electron and molecular geometries