supplemental digital content 3
advertisement

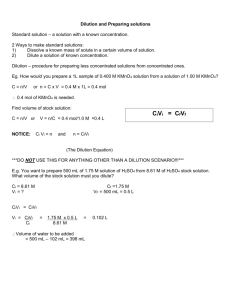
SUPPLEMENTAL DIGITAL CONTENT 3 Materials Isolated rat hearts were perfused with Krebs-Henseleit solution (NaCl 118.0 mM; KCl 4.7 mM; MgSO4 1.2 mM; KH2PO4 1.2 mM; NaHCO3 25.0 mM; CaCl2 2.5 mM and glucose 11.0 mM, in MilliQ water) maintained at 37°C. This perfusate was bubbled continuously with Carbogen (95% O2 - 5% CO2) for an hour, resulting in a final pH of between 7.3 and 7.4. The perfusate was filtered through an in line filter (5 µm) during both pre and post-storage reperfusion. Celsior solution was used for both arresting and storing the hearts. rh-NRG-1 (Zensun Sci & Tech Co. Ltd., Shanghai, China) (14nM) was added to the perfusate (37◦C) and/or C (4◦C) at the commencement of the experiment. Cariporide (HOE642) (Aventis Pharma, Germany) (10µM) and GTN (BDL, Australia) (0.1 mg/ml) were added to the C solution immediately before use. Doses of agents used were based on previous experiments performed in our laboratory and also on effective rhNRG-1 doses used in various animal models of cardiomyopathy (4, 8, 17). Rats were anaesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Isolated Working Heart Model Aortic pressure was monitored via a side arm of the aortic cannula with a pressure transducer (Ohmeda, Pty Ltd., Singapore). Aortic flow was measured via an in line flow meter (Transonics Instruments Inc. Ithaca, NY). Aortic pressure and flow were recorded using MacLab/4e (ADInstruments Pty Ltd, Sydney, Australia) and heart rate was calculated from the flow trace. Coronary flow was measured by timed collection of the coronary effluent draining from an incision in the pulmonary artery. Immunohistochemical and histological analysis Briefly, antigens were retrieved using a Novocastra Epitope Retrieval Solution (pH6.0, 10x concentrate) before washing in Bond Wash Solution (10x concentrate, Leica Biosystems, Newcastle Upon Tyne, U.K) and soaking in 3% hydrogen peroxide. Proteins were blocked using a Novolink Polymer Detection System (Novocastra, Newcastle Upon Tyne, U.K). Slides were then soaked in primary antibody solution (cleaved Caspase 3 rabbit anti human/rat antibody (Asp175), 1:50 dilution, Cell Signaling Technologies) at 4◦C overnight. After washing, post primary block and polymer application, slides were stained with 3,3'-Diaminobenzidine and Harris haematoxylin. Digital images were acquired at 600x magnification. Ten representative images of each slide were acquired for quantification of 3,3'-Diaminobenzidine (cleaved caspase 3) staining using Image J (Version 1.41, National Institutes of Health, USA) (36). Western blotting. Sixty milligrams of tissue from each of three hearts in all groups were homogenized in ice-cold lysis buffer (150 mM NaCl, 50 mM Tris HCl, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM β-glycerophosphate, 5 mM dithiothreitol, 15% Roche cocktail protease inhibitors, pH 7.4). Samples were then centrifuged at 10,000 rpm for 5 min at 4°C. Protein concentration of each lysate was measured using a Bradford assay kit (Pierce Biotechnology Inc, Rockford, IL), with bovine serum albumin as the standard. Protein samples were boiled in sample loading buffer for 5 min before loading onto 8% SDS-polyacrylamide gel (30 µg per lane). After fractionation on a Protean III system (BioRad Laboratories, Regents Park, Australia), proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Australia). Membranes were blocked for 1 hr in Tris buffered saline, (pH 7.4) containing 1% bovine serum albumin and 0.1% Tween 20. Membranes were probed overnight with rabbit polyclonal antibodies raised against total and phospho Akt (Ser 473; 1:1000 dilution), total and phospho GSK-3B (Ser 9; 1:1000 dilution) and total and phospho STAT3 (Tyr 705; 1:1000 dilution) (Cell Signaling Technology, Danvers, MA). A mouse antibody was used to probe for total and phospho ERK (Thr 202/Tyr 204; 1:1000 dilution). The secondary antibodies were horseradish peroxidise conjugated anti-rabbit and anti-mouse immunoglobulin G, respectively (Amersham Life Sciences). Protein bands were visualized by enhanced chemiluminescence (Amersham Life Sciences), digitized, and then quantified using Image J (Version 1.41, National Institutes of Health, USA).