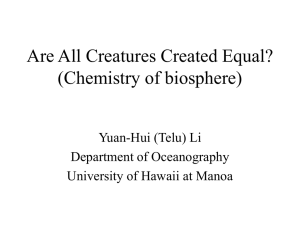
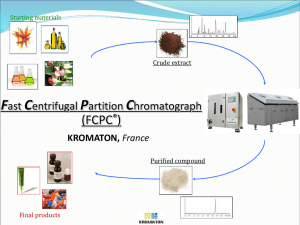
Journal: Reaction Kinetics, Mechanisms and Catalysis
advertisement

ELECTRONIC SUPPLEMENTARY MATERIAL Title: Determination of methanolysis rate constants for low and high fatty acid oils using heterogeneous surface reaction kinetic models Journal: Reaction Kinetics, Mechanisms and Catalysis Author Names & Affiliations: Yousuf Jamal Institute of Environmental Sciences and Engineering, School of Civil and Environmental Engineering, National University of Sciences and Technology, H-12, Islamabad 44000, Pakistan Ahmed Rabie Department of Petroleum Engineering, Texas A&M University, College Station, TX 77843, USA. Bryan Boulanger Department of Civil Engineering, Ohio Northern University, Ada, OH 45810, USA Corresponding author email address: yousuf.jamal@iese.nust.edu.pk 1 ELEY RIDEAL REACTION MECHANISM REACTION: A+B ßà C+D One or more reactants are not surface adsorbed B B B D A A A 1 Catalyst Surface C 2 3 B moved from bulk solution to A A Adsorbed D C Surface reaction D C 4 Reaction site on the catalyst surface Desorption 5 Desorption Fig. S1 Graphical depiction of the reaction mechanism involved in the EleyRideal kinetic model. 2 LANGMUIR HINSHELWOOD HOUGEN WATSON REACTION MECHANISM REACTION: A+B ßà C+D All the reactants are surface adsorbed before reaction. B B A A A B 2 1 Catalyst Surface B Adsorbed A Adsorbed C C D D 4 3 Surface reaction D C Desorption 5 Desorption Reaction site on the catalyst surface Fig. ESM 2 Graphical depiction of the reaction mechanism involved in the Langmuir HinshelwoodHougenWatson kinetic model. 3 APPENDIX A ER surface reaction model derivation Based upon the assumptions provided, the following series of equations can be derived for ER surface reaction model solution. The stepwise reactions in the ER model derivation include: k1 Step 1: MeOH +∗ →← MeOH ∗ Step 2: T + MeOH ∗ Step 3: D + MeOH ∗ Step 4: M + MeOH ∗ Step 5: D +∗ →← D∗ Step 6: M +∗ →← M∗ Step 7: G +∗ →← G∗ k_1 k2 → ← k_2 k3 → ← k_3 k4 → ← k_4 (1) E + D∗ (2) E + M∗ (3) E + G∗ (4) k5 k_5 (5) k6 k_6 (6) k7 k_7 (7) where: MeOH = methanol; T = triglyceride; E = methyl ester; G = glycerol; * = resin surface site; and MeOH*, T*, E*, D*, M* and G* are bounded resin surface sites; kn=forward reaction; k-n= backward reaction, n = reaction step 4 Note: K Adsorption = kforward kbackward and K k Desorption = backward kforward Therefore, the rate equations for the adsorption of methanol and the surface reaction of adsorbed MeOH with T, D, M, and G can be written as follows: Methanol adsorption as rate limiting step r1 = k1 [MeOH][∗] − k −1 [MeOH ∗ ] K1 = (8) k1 k −1 1 r1 = k1 ([MeOH][∗] − K [MeOH ∗ ]) 1 (8a) Because MeOH adsorption is the rate limiting step, k2 through k7 will be much larger than r r r r r r k1. Therefore, k2 , k3 , k4 , k5 , k6 and k7 in the following series of equations are assumed to be 2 3 4 5 6 7 very small (almost zero). Rate of triglycerides surface reaction r2 = k 2 [MeOH ∗ ][T] − k −2 [D∗ ][E] K2 = (9) k2 k −2 5 r2 k2 1 ≅ 0 = ([MeOH ∗ ][T] − K [D∗ ][E]) 2 (9a) Rate of diglycerides surface reaction r3 = k 3 [MeOH ∗ ][D] − k −3 [M ∗ ][E] K3 = r3 k3 (10) k3 k −3 1 ≅ 0 = ([MeOH ∗ ][D] − K [M ∗ ][E]) 3 (10a) Rate of monoglycerides surface reaction r4 = k 4 [MeOH ∗ ][M] − k −4 [G∗ ][E] K4 = r4 k4 (11) k4 k −4 1 ≅ 0 = ([MeOH ∗ ][M] − K [G∗ ][E]) 4 (11a) Rate of diglycerides desorption: r5 = k 5 [D][∗] − k −5 [D∗ ] K5 = (12) k5 k −5 6 r5 k5 1 ≅ 0 = ([D][∗] − K [D∗ ]) 5 [D ∗] = K 5 [D][∗] (12a) Rate of monoglycerides desorption: r6 = k 6 [M][∗] − k −6 [M ∗ ] K6 = r6 k6 (13) k6 k −6 1 ≅ 0 = ([M][∗] − K [M ∗ ]) 6 [M ∗] = K 6 [M][∗] (13a) Rate of glycerol desorption: r7 = k 7 [G][∗] − k −7 [G∗ ] K7 = r7 k7 (14) k7 k −7 1 ≅ 0 = ([G][∗] − K [G∗ ]) [G ∗] = K 7 [G][∗] 7 (14a) 7 Solving for [MeOH*] in equations (9a-14a) 1 [G∗ ][E] [MeOH ∗ ] = K 1 K7 [G][E] [∗] [M] 4 =K [M] 4 1 [M∗ ][E] [MeOH ∗ ] = K 1 K6 [M][E] [∗] =K [D] 3 1 [D∗ ][E] [MeOH ∗ ] = K Therefore, (16) [D] 3 1 K5 [D][E] [∗] [T] 2 =K [T] 2 (15) 1 K7 [G] [MeOH ∗ ] = (K 4 [M] (17) 1 K6 [M] =K 3 [D] 1 K5 [D] =K 2 [T] ) [E] [∗] (18) The equation that represents the overall mass balance on the surface sites is: ST = [∗] + [MeOH ∗ ] + [D∗ ] + [M ∗ ] + [G∗ ] (19) where ST is the total binding sites on the surface. Considering [MeOH ∗ ] = 1 K5 [D][E] [∗] K2 [T] (20) and substituting [MeOH*] into the site balance equation 1 K5 [D][E] [∗] ST = ([∗] + (K [*] = 2 [T] ) + K 5 [D][∗] + K 6 [M][∗] + K 7 [G][∗]) ST 1 K5 [D][E] ([1]+ ( K2 [T] )+ K5 [D] +K6 [M] +K7 [G]) (21) (22) 8 Rate of methanol adsorption from equation 8a 1 r1 = k1 ([MeOH][∗] − K [MeOH ∗ ]) 1 Substituting in the value of [MeOH*] from equation 20 r1 = k1 ([MeOH][∗] − r1 = k1 ([MeOH] − 1 K5 [D][E] [∗] K1 K2 [T] 1 ( )) 1 1 K 5 [D][E] ( )) [∗] K1 K 2 [T] Substituting in the value of [*] from equation 22 k1 ST ([MeOH]− r1 = ([1]+ ( 1 K5 [D][E] K2 [T] 1 1 K5 [D][E] ( K 1 K2 [T] )) )+ K5 [D] +K6 [M] +K7 [G]) (23) Because the model assumes that methanol adsorption is the rate limiting step, neglecting the presence of intermediates formed during the reaction and combining k1ST into k reduces the model to the following final form shown in equation 24. Rate of methanol consumption r1 = d[MeOH] dt k([MeOH]) 7 [G]) = − ([1]+K (24) For the case of high FA content in soybean oil, an additional term for FFA adsorbed at the resin surface only is added to the model in the following form. 9 Rate of FFA adsorption: r8 = k 8 [FFA][∗] − k −8 [FFA∗ ] K8 = r8 k8 (25) k8 k −8 1 ≅ 0 = ([FFA][∗] − K [FFA∗ ]) 8 [FFA ∗] = K 8 [FFA][∗] (25a) The site balance from equation (21) with this additional term becomes 1 K5 [D][E] [∗] [T] 2 ST = ([∗] + (K ) + K 5 [D][∗] + K 6 [M][∗] + K 7 [G][∗] + K 8 [FFA][∗]) (26) which represents the addition of one extra term to the derivation of the methanol adsorption: r1 = − kMeOH ST ([MeOH]− ([1]+ ( 1 K5 [D][E] K2 [T] 1 1 K5 [D][E] ( Keq K2 [T] )) )+ K5 [D] +K6 [M] +K7 [G]+K8 [FFA]) (27) Therefore, equation 28 represents the final derived form of the ER model with FFA present when k1ST is combined into k and all assumptions are considered: r1 = d[MeOH] dt =− k([MeOH]) ([1]+K7 [G]+K8 [FFA]) (28) 10 APPENDIX B LHHW surface reaction model derivation Based upon the assumptions provided, the following series of equations can be derived for the LHHW surface reaction model solution. The stepwise reactions in LHHW model derivation include: k1 Step 1: MeOH +∗ →← MeOH ∗ (1) k–1 k2 Step 2: T +∗ → ← T∗ (2) k–2 Step 3: T ∗ + MeOH ∗ k3 → ← E ∗ + D∗ (3) E∗ + M∗ (4) E ∗ + G∗ (5) k–3 Step 4: D∗ + MeOH ∗ k4 → ← k–4 Step 5: M ∗ + MeOH ∗ k5 → ← k–5 k6 Step 6: E +∗ →← E ∗ (6) k–6 k7 Step 7: D +∗ →← D∗ (7) k–7 k8 Step 8: M +∗ →← M ∗ (8) k–8 11 k9 G +∗ →← G∗ Step 9: (9) k–9 where: MeOH = methanol; T = triglyceride; E = methyl ester; G = glycerol; * = resin surface site; and MeOH*, T*, E*, D*, M*, and G* are bounded resin surface sites; kn=forward reaction; k-n= backward reaction, n = reaction step Note: K Adsorption = and kforward kbackward K k Desorption = backward kforward Therefore, the rate equations for the adsorption of methanol and the surface reaction of adsorbed MeOH with T, D, M, and G can be written as follows: Methanol adsorption as rate limiting step r1 = k1 [MeOH][∗] − k −1 [MeOH ∗ ] K1 = (10) k1 k −1 1 r1 = k1 ([MeOH][∗] − K [MeOH ∗ ]) (10a) 1 Because MeOH adsorption is the rate limiting step, k2 through k9 will be much larger than r r r r r r r r k1. Therefore, k2 , k3 , k4 , k5 , k6 , k7 , k8 , and k9 in the following series of equations are assumed 2 3 4 5 6 7 8 9 to be very small (almost zero). Rate of triglycerides adsorption: 12 r2 = k 2 [T][∗] − k −2 [T ∗ ] K2 = r2 k2 (11) k2 k −2 1 ≅ 0 = ([T][∗] − K [T ∗ ]) (11a) 2 Rate of surface reaction of bound triglyceride with bound methanol r3 = k 3 [MeOH ∗ ][T ∗ ] − k −3 [D∗ ][E ∗ ] K3 = r3 k3 (12) k3 k −3 1 ≅ 0 = ([MeOH ∗ ][T ∗ ] − K [D∗ ][E ∗ ]) 3 (12a) Rate of diglycerides surface reaction r4 = k 4 [MeOH ∗ ][D∗ ] − k −4 [M ∗ ][E ∗ ] K4 = r4 k4 (13) k4 k −4 1 ≅ 0 = ([MeOH ∗ ][D∗ ] − K [M ∗ ][E ∗ ]) 4 (13a) Rate of monoglyceride surface reaction 13 r5 = k 5 [MeOH ∗ ][M ∗ ] − k −5 [G∗ ][E ∗ ] K5 = r5 k5 (14) k5 k −5 1 ≅ 0 = ([MeOH ∗ ][M ∗ ] − K [G∗ ][E ∗ ]) 5 (14a) Rate of methyl ester desorption r6 = k 6 [E][∗] − k −6 [E ∗ ] K6 = r6 k6 (15) k6 k −6 1 ≅ 0 = ([E][∗] − K [E ∗ ]) 6 [E ∗] = K 6 [E][∗] (15a) Rate of diglycerides desorption r7 = k 7 [D][∗] − k −7 [D∗ ] K7 = r7 k7 (16) k7 k −7 1 ≅ 0 = ([D][∗] − K [D∗ ]) 7 14 [D ∗] = K 7 [D][∗] (16a) Rate of monoglycerides desorption r8 = k 8 [M][∗] − k −8 [M ∗ ] K8 = r8 k8 (17) k8 k −8 1 ≅ 0 = ([M][∗] − K [M ∗ ]) 8 [M ∗] = K 8 [M][∗] (17a) Rate of glycerol desorption r9 = k 9 [G][∗] − k −9 [G∗ ] K9 = r9 k9 (18) k9 k −9 1 ≅ 0 = ([G][∗] − K [G∗ ]) 9 [G ∗] = K 7 [G][∗] (18a) Solving for [MeOH*] in equations (11a-18a) 15 [MeOH ∗ ] = K9 K6 [G][E][∗] [MeOH ∗ ] = K6 K8 [M][E][∗] [MeOH ∗ ] = K6 K7 [D][E][∗] K5 K8 [M] = = K4 K7 [D] K3 K2 [T] = K9 K6 [G][E][∗] (19) K5 K8 [M] K6 K8 [M][E][∗] (20) K4 K7 [D] K6 K7 [D][E][∗] (21) K3 K2 [T] K K [G] Therefore [MeOH ∗ ] = (K 9K 6[M] = 5 8 K6 K8 [M] K4 K7 [D] = K6 K7 [D] ) [E][∗] K3 K2 [T] The equation that represents the overall mass balance on the surface sites is: ST = [∗] + [MeOH ∗ ] + [T ∗ ] + [E ∗ ] + [D∗ ] + [M ∗ ] + [G∗ ] (22) where ST is the total binding sites on the surface. Considering [MeOH ∗ ] = K6 K7 [D][E][∗] (23) K3 K2 [T] and substituting [MeOH*] into the site balance equation ST = ( [*] = K6 K7 [D][E][∗] K3 K2 [T] [∗] + ( ) + k 2 [T][∗] + k 6 [E][∗] + k 7 [D][∗] +k 8 [M][∗] + k 9 [G][∗] ST K K [D][E] ([1]+ ( 6 7 [T] K3 K2 )+ k2 [T]+ k6 [E]+k7 [D] +k8 [M] +k9 [G]) ) (24) (25) 16 Rate of methanol adsorption from equation 10a is 1 r1 = k1 ([MeOH][∗] − K [MeOH ∗ ]) 1 Substituting in the value of [MeOH ∗ ] from equation 23 r1 = k1 ([MeOH][∗] − 1 K1 1 ( r1 = k1 ([MeOH] − K ( 1 K6 K7 [D][E][∗] K3 K2 [T] )) K6 K7 [D][E] )) [∗] K3 K2 [T] Substituting in the value of [*] from equation 25 k1 ST ([MeOH]− r1 = 1 K6 K7 [D][E] ( )) K1 K3 K2 [T] K K [D][E] ([1]+( 6 7 [T] )+ K2 [T]+ K6 [E]+K7 [D] +K8 [M] +K9 [G] ) (26) K3 K2 Because the model assumes that methanol adsorption is the rate limiting step, neglecting the presence of intermediates formed during the reaction and combining k1ST into k reduces the model to the following final form shown in equation 27. r1 = d[MeOH] dt =− k([MeOH]) ([1]+K2 [T]+K6 [E]+K9 [G]) (27) For the case of high FFA content in soybean oil, an additional term for FFA adsorbed at the resin surface only is added to the model in the following form. 17 Rate of FFA adsorption: r10 = k10 [FFA][∗] − k −10 [FFA∗ ] k10 k −10 K10 = r10 k10 (28) 1 ≅ 0 = ([FFA][∗] − K [FFA∗ ]) 10 [FFA ∗] = K10 [FFA][∗] (28a) The site balance from equation (24) with this additional term becomes ST = ( K6 K7 [D][E][∗] K3 K2 [T] [∗] + ( ) + K 2 [T][∗] + K 6 [E][∗] + K 7 [D][∗] + K 8 [M][∗] +K 9 [G][∗] + K10 [FFA][∗] ) (29) Rearranging eq (29) [*] = ST K K [D][E] ([1]+ ( 6 7 [T] K3 K2 )+ K2 [T]+ K6 [E]+K7 [D] +K8 [M] +K9 [G]+K10 [FFA]) (30) which represents the addition of one extra term to the derivation of the methanol adsorption: 18 r1 = − k1 ST ([MeOH]− 1 K6 K7 [D][E] ( )) Keq K3 K2 [T] K K [D][E] ([1]+( 6 7 [T] )+ K2 [T]+ K6 [E]+k7 [D] +K8 [M] +K9 [G]+ K10 [FFA]) (31) K3 K2 Therefore, equation 32 represents the final derived form of the LHHW model with FFA present when k1ST is combined into k and all assumptions are considered: r1 = d[MeOH] dt =− k([MeOH]) ([1]+K2 [T]+K6 [E]+K9 [G]+K10 [FFA]) (32) 19