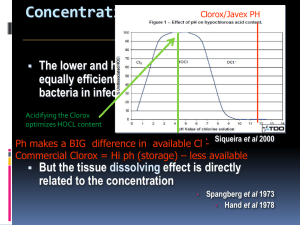
DETERMINING MOST EFFECT METHOD OF WATER
advertisement

DETERMINING MOST EFFECT METHOD OF WATER STERILIZATION BY MEASURING BACTERIAL CONCENTRATIONS Meghan Maltby, Victor Longoria, Hanna M. Fard Department of Biological Science Saddleback College Mission Viejo, CA, 92692 Abstract: Water contamination is a problem in many developing countries. This experiment tested the reliability of four methods of water sterilization on water samples collected from Mission Viejo Lake. The methods were: boiling bath, Clorox™, iodine, and SteriPen™. All were predicted to have the same effect on sample quality. Multi-tube fermentation was used to determine MPN for pre-treatment samples, averaging to 460. After sterilization, MPN could not be calculated due to lack of gas production. Multi-tube fermentation of the post-treatment samples showed boiling water had the second least amount of acid production at 36% of tubes and least amount of colony growth at 11%. Clorox™ showed 33% and 14%, iodine showed 58% and 81%, and SteriPEN™ showed 50% and 39%, respectively. Post-treatment samples were streaked onto NA plates where a fourth of boiling showed growth, two from Clorox™ treatment, and all four from iodine and SteriPEN™. Gram stains were done from the samples that showed growth of which all contained Gram negative bacteria. Only boiling and Clorox™ treatment samples shows presence of Gram negative bacteria types. These results gave very strong evidence that boiling water is the sterilization technique out of the four that were tested. Introduction: Water contamination is a problem when traveling to developing countries especially when camping in remote areas where water is crucial. Sterilization techniques such as boiling water, chlorination, and iodination have been used for decades to cleanse water for drinking. The oldest method of water sterilization is boiling water, which is simple and effective. Boiling water for 3 minutes and letting it cool has been shown as the ideal method for water purification (Oldham et al., 2008). Chlorination is another basic sterilization method that has proven successful. A few drops of chlorine or Clorox™ into half a liter of water can provide drinking water in 30 minutes. For decades, chlorine has proven the best in “removing non-spore forming bacteria indicators such as Escherichia coli and fecal enterococci,” (Montemayor et al., 2008). Iodine has the same procedure as chlorine to sterilization water. Within 30 minutes of adding a few drops of iodine into a contaminated water one can have sufficient drinking water. Iodine and chlorine are also used by medical clinics in developing countries chlorine as sterilization techniques for certain medical equipment. Iodine tablets have been used for years in the military. The use of iodine tablets have proven useful due to “the biocidal efficacy of iodine against protozoa Giardia muris,” (Starke et al., 2005). SteriPen™ is the fourth sterilization technique being tested that uses ultraviolet lighting to destroy bacteria, viruses, and protozoa. A recent study showed that ultraviolet sterilizers are a “suitable replacement of chlorine disinfection in the removal of microbiological contaminants in domestic water supply” (Adegbola & Olaoye, 2012). Contaminated water was collected in four different containers from Mission Viejo Lake and all four water purification techniques were used to sterilize a portion of each sample. Methods: Four samples from Mission Viejo Lake were collected, two from dusk and two from the next morning. On each sample multi-tube fermentation was completed to determine Most Probable Number (or MPN) for bacterial concentration. Ten ml of a sample was transferred into three separate triple strength tubes of lactose with brom-thymol blue. One ml of the water sample was transferred into three regular strength lactose tubes and 0.1 mL of sample was transferred into three separate regular strength lactose tubes. The tubes were incubated for 48 hours and were examined for gas and acid production. If both gas and acid production were present, the presumptive test was positive. If neither or only one of the changes were present the presumptive test was negative. The number of positive versus negative presumptive tests was used to determine the MPN. The confirm test was completed using test tubes that showed more than 10% gas production. A loopful of broth from one positive presumptive lactose tube for each water sample was aseptically streaked onto an EMB agar plate using isolation streaking technique. EMB plates were incubated then examined for coliform bacterial growth and E. coli presence. A Gram stain was completed using a single isolated colony from the EMB plates to determine presence of Gram negative or Gram positive bacteria. The remainders of the water samples collected were subjected to four water sterilizations: boiling bath, iodine treatment, chlorination, and SteriPEN™. For the boiling bath, 300 mL of sample was placed in a glass container and heated until boiling. The sample was boiled for 10 minutes and then cooled. The SteriPEN™ sterilization was conducted by placing 300 mL of sample in a large container and placing the tip of the SteriPEN™ in the sample, turning on the pen, and stirring for 90 seconds while the UV light was on. The iodine sterilization was conducted by adding 2 drops (~0.1 mL) of iodine tincture into 300 mL of sample. After stirred thoroughly the test stood for 30 minutes. The chlorine sterilization was conducted by adding 2 drops (~0.1 mL) of Clorox™ into 300 mL of the water sample and after stirred thoroughly, stood for 30 minutes. After sterilization mutli-tube fermentation was done for each test of each sample using the same procedure as stated above. The incubated lactose tubes were examined and a loopful of broth from one tube out of each multi-tube fermentation, excluding the initial test, was aseptically streaked using streak plate technique onto nutrient agar plates. After incubation the plates showing growth were Gram stained and examined for the presence of Gram negative or Gram positive bacteria, as well as shape and size of the bacteria. Six of the plates showing growth were chosen based on opacity, margin, moisture content, and pigmentation to be aseptically streaked on MacConkey plates using steak plate technique to determine if the colonies were lactose fermenting or not. Results: The multi-tube fermentation tests for the unsterilized initial samples averaged to be 420.25 bacterial concentration per 100 mL of sample. Prior to sterilization the remainder of the samples had a small bit of sediment settling to the bottom of the containers as well as a notorious “lake water” smell. After sterilization some of these factors changed. After the four samples were boiled, the water still had the sediment and the lake water smell, but three out of four samples had accumulated a very slightly opaque, colorless film on the top. After the iodine tincture treatment the samples still had the sediment in the water and one sample smelled clean, one of lake water, and two very faintly of iodine. After the Clorox™ treatment all samples smelled slightly of bleach and had sedimentation. After the SteriPEN™ treatment the samples smelled only very faintly of lake water. The multi-tube fermentation tests for the four purification treatments returned results of acid production and colony growth, but not a single tube showed gas production. Thus, each type of sterilization destroyed enteric bacteria. However, as shown in Figures 1 and 2, 36% of lactose tubes after boiling treatment showed acid production and 11% showed colony growth. Iodine treatment tubes showed 58% acid production and 81% growth, while Clorox treatment tubes showed 33% acid production and 14% growth, and SteriPEN™ tubes showed 50% acid production and 39% growth. Out of the four boiling treatment samples streaked onto NA plates only one showed colony growth. Clorox treatment had two plates of growth, and both iodine and SteriPEN™ treatments had all four plates showing growth. The plates showing growth were Gram stained which showed that the one plate grown from the boiling sample contained both Gram positive and Gram negative bacteria. The Clorox treatment plates also showed signs of both Gram positive and Gram negative bacteria. Both iodine and SteriPEN™ showed only signs of Gram negative bacteria. The MacConkey tests for the six samples showed only one colony to be lactose fermenting, which was from a sample sterilized with Clorox. The other sample sterilized with Clorox showed growth on the MacConkey plate, but the colony was not lactose fermenting. Three plates of MacConkey showed growth, but lactose fermenting, and one plate had no growth at all. Figure 1: Acid Production in Multi-Tube Fermentation Tests. This graph shows the percentages of acid production seen in the multi-tube fermentation lactose tubes for the four different sterilization methods. Figure 2: Visible Colony Growth in Multi-Tube Fermentation Tests. This figure shows the percentage tubes in the multi-tube fermentation tests for each treatment method that show visible colony growth. Discussion: Four water samples were tested using four methods of sterilization: boiling bath, chlorination, iodination, and SteriPEN™. Multi-tube fermentation allowed a closer look into the types of bacteria affected by the sterilization techniques. Each sterilization method showed acid production and colony growth, but no gas production was seen in any of the lactose tubes. The absence of gas production provides evidence that enteric bacteria were completely wiped out. Figure 1 shows the percentage of lactose tubes that showed both acid production and colony growth for each of the different types of sterilization. When averaged out, boiling water had the least amount of acid production and colony growth within the tubes. Along with that, of the four plates streaked with sample treated by boiling bath, only one showed colony growth. The advantages of decontamination of water with boiling baths are that the materials are easily found and are readily available and more often than not, cheap. However, boiling water can be time consuming when one needs clean water fast. Materials that are suspended in the water will stay there and even after treatment will continue to make the water look murky as well as make the water smell. Treatment with Clorox™ had similar results from the fermentation. When averaged, chlorination treatment had the second least amount of acid production and colony growth, both just a few digits away from boiling percentages. The advantages of Clorox™ treatment is that water will smell clean while being treated and it will kill enteric bacteria. However, it is easy to incorrectly measure treatment and add too much Clorox™ to water and make it unsuitable for ingestion. Also, chlorination treatment will cause the water to smell slightly of bleach and it may possibly not have an effect on Gram negative bacteria, which are the types of bacteria that are more resistant to antibiotic treatment. Iodine tincture treatment fared low along with sterilization by SteriPEN™. Iodine had the worst percentages for the fermentation tests and all plates that were streaked with what was supposed to be clean water grew large amounts of bacteria. Iodine took care of the enteric bacteria, but its range after that seemed limited. SteriPEN™ treatment was the least time consuming of all techniques but fared second to worst. The advantage of SteriPEN™ is that it is a compact device easily carried around when traveling, but that amount of bacteria that it decimates seems lower than that of a boiling bath of Clorox™ treatment. The device is also very expensive, which makes it a novelty. Another advantage of the SteriPEN™ was that the sample smelled very clean after use, which gave the illusion of being clean drinking water. The most effective method of water purification is a boiling bath. The numbers for acid production and colony growth are by far the lowest and the advantages of having the least contaminated water outweigh the disadvantage of time. It is recommended that water is filtered before being boiled, to ensure that no suspended materials that are big enough to be caught by a filter remain in the water. Works Cited Adegbola, A., & Olaoye, R. (2012). Investigating the Effectiveness of Ultraviolet (UV) Water Purification as Replacement of Chlorine Disinfection in Domestic Water Supply. International Journal Of Engineering Science & Technology, 4(8), 3891-3897. Montemayor, M. M., Costan, A. A., Lucena, F. F., Jofre, J. J., Muñoz, J. J., Dalmau, E. E., & ... Sala, L. L. (2008). The combined performance of UV light and chlorine during reclaimed water disinfection. Water Science & Technology, 57(6), 935-940. doi:10.2166/wst.2008.206 Oldham, D., Crawford, P., & Nichols, W. (2008). What is the best portable method of purifying water to prevent infectious disease? (Cover story). Journal Of Family Practice, 57(1), 4648. Sodha, S. V., Menon, M. M., Trivedi, K. K., Ati, A. A., Figueroa, M. E., Ainslie, R. R., & ... Quick, R. R. (2011). Microbiologic effectiveness of boiling and safe water storage in South Sulawesi, Indonesia. Journal Of Water & Health, 9(3), 577-585. doi:10.2166/wh 2011.255 Starke, J., Bowman, D., Labare, M., Fogarty, E., Lucio-Forster, A., Barbi, J., Jenkins, M., & Pavlo, M. (2005). Do iodine water purification tablets provide an effective barrier against cryptosporidium parvum?. Military Medicine, 170(1), 83-86. Zemlyn, Seymour, William W. Wilson, and Paul A Hellweg. "A Caution on Iodine Water Purification." Western Journal of Medicine. 135 (1981): 166-167. Web. 12 Nov. 2013. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1273058/>.