Aqueous Solutions Water is the dissolving medium, or solvent
advertisement

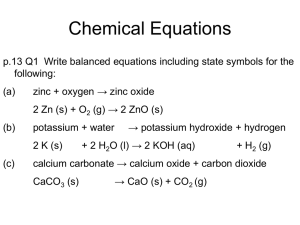
Aqueous Solutions Some Properties of Water: Water is the dissolving medium, or solvent. Water is able to dissolve so many substances because: Water Water is “bent” or V-shaped. The O-H bonds are covalent. Water is a polar molecule. Hydration occurs when salts dissolve in water. is a polar molecule because it is a bent) is a partial charge--less than 1. 04_40 H 2 105 O H + Water is a polar molecule because it is a bent molecule. The hydrogen end is while the oxygen end is - Delta () partial charge--less than 1. SOLUTE: - dissolves in water (or other “solvent”) - changes phase (if different from the solvent) - is present in lesser amount (if the same phase as the solvent) SOLVENT: - retains its phase (if different from the solute) - is present in greater amount (if the same phase as the solute) 04_41 + + + + + H O H + Cation + + + + + H H O + + Anion Polar water molecules interact with the positive and negative ions of a salt, assisting in the dissolving process. This process is called hydration. SOLUBILITY: The general rule for solubility is: “Like dissolves like.” Polar water molecules can dissolve other polar molecules such as alcohol and, also, ionic substances such as NaCl. Non-polar molecules can dissolve other non-polar molecules but not polar or ionic substances. Gasoline can dissolve grease. How does the rule “Like dissolves like.” apply to cleaning paint brushes used for latex paint as opposed to those used with oil-based paint? MISCIBILITY: Miscible -- two substances that will mix together in any proportion to make a solution. Alcohol and water are miscible because they are both polar and form hydrogen bonds. Immiscible -- two substances that will not dissolve in each other. Oil and vinegar are immiscible because oil is non-polar and vinegar is polar. *Solubility Rules for ionic compounds: Compounds containing the following ions are generally soluble in water. 1) 2) 3) 4) 5) 6) alkali metal ions and ammonium ions (Li+, Na+, K+, NH4+) acetate ions (C2H3O2-) nitrate ions (NO3-) chlorate ions (ClO3-) halide ions (Cl-, Br-, I-) (except for silver, mercury (I) and lead (II), which are insoluble) sulfate ion (SO4-2) )except for strontium, barium and lead (II), which are insoluble) Compounds containing the following ions are only slightly soluble in water. 7) 8) 9) 10) 11) carbonate ions (CO3-2) (see rule 1 for exceptions) chromate ions (CrO4-2) (see rule 1 for exceptions) phosphate ions (PO4-2) (see rule 1 for exceptions) sulfide ions (S-2) (calcium, barium, lead II and rule 1 exceptions are soluble) hyrdoxide ions (OH-) (calcium, barium, strontium and rule 1 exceptions are soluble) Electrolytes & Non-electrolytes: An electrolyte is a material that dissolves in water to give a solution that conducts an electric current. A non-electrolyte is a substance which, when dissolved in water, gives a non-conducting solution. ELECTROLYTES: Strong - conduct current efficiently and are soluble salts, strong acids, and strong bases. NaCl, KNO , HNO , NaOH 3 3 Weak - conduct only a small current and are weak acids and weak bases. HC H O , aq. NH , tap HOH 2 3 2 3 Non - no current flows and are molecular substances pure H O, sugar solution, glycerol 2 Power Source 04_43 + + + + + + (a) (b) (c) Electrical conductivity of aqueous solutions. a) strong electrolyte b) weak electrolyte c) nonelectrolyte in solution. Svante Arrhenius first identified these electrical properties. 2+ - When BaCl dissolves, the Ba and Cl ions are randomly 2 dispersed in the water. BaCl is a strong electrolyte. 2 04_1529 BaCl2(s) dissolves = Ba2+ = Cl . ACIDS: + Strong acids - dissociate completely (~100 %) to produce H in solution HCl, H SO , HNO , HBr, 2 HI, & HClO 4 4 3 + Weak acids - dissociate to a slight extent (~ 1 %) to give H in solution HC H O , HCOOH, HNO , & 2 H SO 2 3 2 3 04_1530 + + + + + + + + + + + = H+ = Cl HCl is completely ionized and is a strong electrolyte. BASES: - Strong bases - react completely with water to give OH ions. sodium hydroxide + NaOH (s) → Na - (aq) + OH - (aq) Weak bases - react only slightly with water to give OH ions. ammonia + NH 3(aq) + HOH ↔ NH (l) 4 (aq) - + OH (aq) 04_1531 + + + + + + + + + + - = OH + = Na+ %. An aqueous solution of sodium hydroxide which is a strong bases dissociating almost 100 %. 2 04_1532 Acetic acid(CH COOH) exists in water mostly as undissociated molecules. Only a small percent of 3 the molecules are ionized. Strength of Solution (Molarity): moles of solute per liter of solution moles of solute per volume of solution in cubic decimeters Molarity (M) = mol/L 1 L = 1 dm3 Molarity units written as: M = mol dm-3 Strength of Solution Calculations: 1. Calculate the strength of a solution prepared by dissolving 11.5 g of solid NaOH in enough water to make 1.50 dm3 of solution. 2. Calculate the strength of solution prepared by dissolving 1.56 of gaseous HCl in enough water to make 26.8 cm3 of solution. 3. How many moles of nitrate ions are present in 25.00 cm3 of a 0.75 mol*dm-3 Co(NO3)2 solution? 4. Typical blood serum is about 0.14 mol*dm-3 NaCl. What volume of blood contains 1.0 mg of NaCl? Standard Solution: a standard solution is a solution whose concentration is accurately known. Standard solutions are made using a volumetric flask: Mass the solute accurately and add it to the volumetric flask Add a small quantity of distilled HOH Dissolve the solute by gently swirling the flask Add more distilled HOH until the level of the solution reaches the etched line on the neck Invert the capped volumetric 25x to thoroughly mix the solution 04_44 Volume marker (calibration mark) Wash Bottle Weighed amount of solute (a) (b) (c) (d) Common terms of solution Concentration: Stock: routinely used solutions prepared in concentrated form Concentrated: relatively large ratio of solute to solvent (5.0 mol*dm-3 NaCl) Dilute: relatively small ratio of solute to solvent (0.01 mol*dm-3 NaCl) Dilution of stock solution: When diluting stock solutions, the moles of solute after dilution must equal the moles of solute before solution (M1V1 = M2V2). Stock solutions are diluted using either a measuring or delivery pipet and a volumetric flask. 04_46 Rubber bulb 500 mL (a) (b) (c) Dilution Calculations: What volume of 16 mol*dm-3 sulfuric acid must be used to prepare 1.5 dm3 of a 0.10 mol*dm-3 H2SO4 solution? Types of Solution Reactions: 1. Precipitation reactions: AgNO3(aq) + NaCl(aq) → NaNO3(aq) + AgCl(s) 2. Acid-Base reactions: NaOH(aq) + HCl(aq) → NaCl(aq) + HOH(l) 3. Oxidation-Reduction reactions: Fe2O3(s) + 2Al(s) 2Fe(s) + Al2O3(s) Cl-(aq) → NO3-(aq) + Na+(aq) + AgCl(s) → Describing Reaction in Solution (refer back to solubility rules): 1. Molecular equation (reactants and products are compounds) AgNO3(aq) + NaCl(aq) → NaNO3(aq) + AgCl(s) 2. Complete ionic equation (all strong electrolytes shown as ions) Ag + (aq) + NO3-(aq) + Na+(aq) + 3. Net Ionic Equation(show only components that actually react – omit spectator ions) Ag + (aq) + Cl-(aq) → AgCl(s) (Na+ and NO3- are spectator ions) Examples: Using the solubility rules, predict what will happen when the following pairs of solutions are mixed. a) KNO3(aq) & BaCl2 (aq) b) Na2SO4(aq) & Pb(NO3)2 (aq) c) KOH(aq) & Fe(NO3)3 (aq) 04_ STOICHIOMETRY FOR REACTIONS IN SOLUTION STEP 1 Identify the species present in the combined solution, and determine what reaction occurs. STEP 2 Write the balanced net ionic equation for the reaction. STEP 3 Calculate the moles of reactants. STEP 4 Determine which reactant is limiting. STEP 5 Calculate the moles of product or products, as required. STEP 6 Convert to grams or other units, as required. Precipitation Calculations: When aqueous solutions of Na2SO4 & Pb(NO3)2 are mixed, PbSO4 precipitates. Calculate the mass of PbSO4 formed when 1.25 dm3 of 0.0500 mol*dm-3 Pb(NO3)2 & 2.00 dm3 of 0.0250 mol*dm-3 Na2SO4 are mixed. 1. 2. 3. 4. Performing Calculations for Acid-Base reactions: 1. List initial species and predict reaction. 2. Write balanced net ionic reaction. 3. Calculate moles of reactants. 4. Determine limiting reactant. 5. Calculate moles of required reactant/product. 6. Convert to grams or volume, as required. What volume of a 0.100 mol*dm-3 HCl solution is needed to neutralize 25.0 cm3 of 0.350 mol*dm-3 NaOH? Key Titration Terms: Titrant - solution of known concentration used in titration Analyte - substance being analyzed Equivalence point - enough titrant added to react exactly with the analyte Endpoint - the indicator changes color so you can tell the equivalence point has been reached. Titration Calculations: 3 If 41.20 cm of NaOH is required to neutralize 1.3009 g of KHP (KHC H O ), what is the 8 4 6 concentration of the NaOH solution? Assume there is one acidic hydrogen in KHP.