Electrophoresis of Amino Acids
advertisement

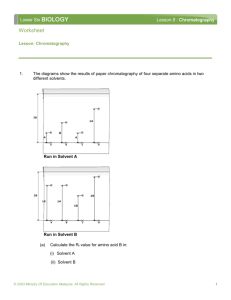
PROTEIN CHROMATOGRAPHY Introduction Chromatography is a technique that is commonly used to separate mixtures of similar substances. A small amount of a sample mixture is placed on a ‘stationary phase’ – in this case paper – while a solvent soaks up the piece of paper. As it does so, the sample will also move, and the distance it moves will depend on the difference between its attraction to the paper and its attraction to the solvent. Molecules that are more attracted to the solvent will move further from their starting point than molecules that are more attracted to the paper. Factors such the size of a molecule, its polarity, its shape, and the presence of ionic groups will all affect how far a molecule moves, and since most molecules have different combinations of these factors, a mixture containing a range of different molecules will be separated into a series of separate spots. Chromatography can be quantified using a measurement called the retention factor R f. Rf is easy to calculate and is simply: 𝑅𝑓 = 𝐷𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑙𝑒𝑑 𝑏𝑦 𝑠𝑝𝑜𝑡 𝐷𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑡𝑟𝑎𝑣𝑒𝑙𝑙𝑒𝑑 𝑏𝑦 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 The retention factor for a molecule should always be the same for a given molecule/solvent/substrate combination, regardless of the size of the chromatography paper and the distance the solvent has moved; this means Rf can be very useful to help us identify an unknown substance. When your mixture is made of coloured substances (for example ink) you can easily see each of the spots. However, many compounds are colourless and so we may need to use techniques such as staining or ultraviolet light to show up the spots. In this experiment you will be using paper chromatography to determine the components present in a mixture of amino acids. Apparatus and Materials Standard laboratory glassware Chromatography paper Ruler Wooden splints Pulled capillary tubes Stapler Hair dryer Ethanol Eluting solution (50:50 propan-1-ol, conc. ammonia) 0.1 M solutions of 4 amino acids, and an ‘unknown’ mixture of two of them. Ninhydrin spray Source: http://www.macalester.edu/~kuwata/Classes/2001-02/Chem%2011/Revised%20Amino%20Acids%20%289%201%2001%29.pdf Procedure 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Before you begin, put on some rubber gloves and clean the ruler and your workspace with ethanol to remove any contaminating proteins. Obtain a strip of a chromatography paper that is approximately 5 x 10 cm, you should still be wearing gloves. Using a pencil (not pen!) mark up the chromatography paper as shown in the diagram. The line should be about 2 cm from the bottom. Take one of the known amino acid solutions and dip a pulled capillary tube into it to suck up some of the solution. Repeatedly and briefly dab the capillary tube on the paper at the cross-mark in line with the letter A; the idea is to build up a small but concentrated spot of the amino acid. Repeat with one of the other solutions on the remaining cross-mark. Allow the spots to dry while you prepare further chromatograms for the remaining two amino acid solutions and for the unknown solution. You may wish to speed this up with a hair dryer. Staple the paper to a wooden splint. In the fume hood, place eluting solvent in deep enough that the chromatography paper will get wet when suspended in the beaker but not so deep that the solvent touches the pencil line. Carefully place your chromatogram in the beaker, keeping it as level as possible and making sure that the paper doesn’t touch the sides. Allow the eluting solvent to soak up the paper. When the solvent front is a few millimetres from the wooden splint, carefully remove it from the beaker, mark the level the solvent reached with a pencil and allow to dry. Spray the paper with ninhydrin spray (in fume hood) to reveal your spots. Use a ruler to measure the total distance travelled by the solvent and the distance travelled by each spot. If you have an unclear spot, measure the position of the top of the spot as this will usually be the best defined part. Record each of the distances and the general shape and definition of the spots. Repeat steps 8-13 with your remaining chromatograms. Analysis a. b. c. d. e. Calculate the retention factors of each of your four amino acids spots. Calculate the retention factors for each of the spots in your unknown sample. Compare your answers to a and b to determine which amino acids were in the unknown sample. Place your amino acids in order from highest to lowest retention factor. Research the structure of each of the amino acids and use your knowledge of the structure of paper, the eluting solvent and intermolecular forces to explain why you got the order outlined in d. Source: http://www.macalester.edu/~kuwata/Classes/2001-02/Chem%2011/Revised%20Amino%20Acids%20%289%201%2001%29.pdf