Growth & Decay
advertisement

Decay is inevitable – how long can we put it off for?
Adrian Oldknow
aoldknow@yahoo.co.uk
It was Benjamin Franklin who said: "... nothing can be said to be certain except death and taxes." Pretty well
any night on television at least one policeman will ask one forensic scientist: “what was the time of death”.
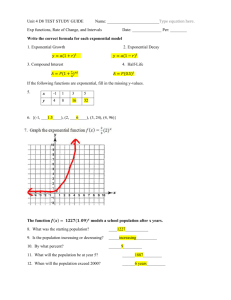
The decay process is a familiar one to us all – and has important implications in, for example, the disposal of
irradiated materials such as spent nuclear fuel rods. It is also the basis of an important technique in modern
archaeology, called radio-carbon dating. In this article we will explore a common mathematical model for
the decay process, called exponential decay – and try to understand what “exponential” means and how it is
related to the strange number e ≈ 2.7183 – the most important mathematical constant after π.
Here’s a simple question for starters – what’s the value of 103? Here we are taking a base value, 10, and
raising it to a power, 3. Another word for a power of a number is its “exponent”. So the process of
exponentiating 10 to the power 3 is to cube it, or to multiply 10 by itself 3 times. So that’s fine – the
answer’s 1000, of course. How about 24 or 0.12? So can we explore the powers of 2, say? Suppose we have
a list of numbers like {1,2,3,4}, then can we raise a base number, like 2, to this list:
2(1,2,3,4}? Try it on a calculator, or TINspire.
The screen below show several base
numbers, 2, 3 and ½, each to two
different sets of exponents. We can see
that 21.5 evaluates to 2.82843, or 2√2 or
√8 . So 21.5 is the same as 23/2 which is
another way to write “the square root of
2 cubed”. Since for any number a>0
there is a value defined for ax we can
explore the graphs of y = ax for different
bases a.
As you can see, the graphs climb from
left to right if a>1 and descend if 1>a>0.
So a graph of an exponential function y =
ax represents a decay if the base a is
between 0 and 1.
We can use a spreadsheet to
generate lists of numbers formed by
repeatedly adding a constant k, and by
repeatedly multiplying by a constant k as
shown below. The value in cell D1 is
stored as the constant k. The rule for A2
is a2 = a1+1, for B2 it is b2 = b1+k, and
for C2 it is c2=c1*k. These formulae are
copied down as far as row 11. Change
the value stored in D1 to change k. The
scatterplots of B against A, and of C
against A are plotted, and a line graph
f5(x) = 1 + k*x, and an exponential graph
f6(x) = 1*kx are drawn through them.
The sequence of numbers in
column B from an additive rule is often called an arithmetic progression and produces linear growth (or
decay), and those in column C from an multiplicative rule are called a geometric progression and produce
exponential growth (or decay).
So, an exponent means a
power, and an exponential function
means a fixed number raised to a
variable power – which is associated
with a multiplicative sequence.
Because we are pretty familiar with
halving and doubling we will make life
simpler by just working with the
growth function: yd (x) = 2x and the
decay function yh (x) = (1/2)x and
applying some simple
transformations.
Now yd (0) = 1 and yd (1) = 2.
Suppose we want an exponential
growth with an initial value of 3 and
which doubles to give a value 6 when
x = 4. If we use y(x) = 3* yd (x) we will be OK through (0,3) – but not through (4,6). Instead we use y(x) = 3*
yd (x/4) = 3*2(x/4). So our general growth function will be yG(x) = a*2(x/b) and decay function yD(x) = a*(1/2)(x/b).
The value of b determines the time taken for a decay to halve in strength – so b is the half-life of the process.
We can now use a simple experiment to test out the ideas. You will need a 9V battery, a resistor (say 100 kΩ
– that is marked with Brown, Black, Yellow and Silver rings), a capacitor (say 200 μF), a voltage probe such as
that which comes with a CBL2 and a Vernier Go!Link (PC) or EasyLink (TI-Nspire handheld) adaptor. The
experiment is described in the Charging Up, Charging Down Classroom Activity at:
http://education.ti.com/calculators/downloads/US/Activities/Detail?id=3989
The voltage data have been captured into a Data
& Statistics page and displayed as a scatterplot.
The Show Exponential Regression function has
been used to see what TI-Nspire’s exponential
regression comes up with! We will use a
Spreadsheet and Graphs to explore how well our
halving and doubling approach agrees. The lists t
and v hold the captured time and voltage data,
and the initial voltage from B1 is stored as the
variable v0 in cell C1. The point H is created to
slide on the x-axis and its coordinates measured.
The x-coordinate of H is stored in the variable h.
The graph of the function f1(x) is for an
exponential decay with a half-life of 15.5 seconds
– just a shade too long. So what is the relationship between our model function 8.363 (1/2)x/15 and the
regression function 8.34* 0.954x? Well we can agree that 8.363 and 8.34 are close enough to v0, but how
are 0.954, ½ and 1/15 related? Try evaluating 0.51/15 - low and behold we get 0.954842. Well h = 15 was
only a rough guess but it looks a pretty close one, doesn’t it?
So in order to study growth and decay we don’t need to know anything about Mr. Naperian’s logarithms, or
the reason e was invented (or was it discovered?). Try doing a similar experiment using a temperature probe
on a cooling liquid. Measure the ambient temperature E of the environment and log the temperature T
against the time t. Then analyse the variable X = T-A to see if you can find its half-life and produce a good
model using exponential decay for the cooling – like Newton did a few years earlier. Now we will see if we
can find the relationship between R, C and the half-life h. You could try using a variable resistor in place of R,
and see how h changes with R for fixed C. Also you could keep R fixed and try some different strength
capacitors. But we’ll go straight for the theoretical approach to see if our experiment confirms it!
From Ohms law we know that V=iR, so the
current i at time t, in amps, is given by i = V/R. In a
spreadsheet we can store the current value of r as
100000, and set up a new column variable i defined
by i = v/r. The scattergram of i against t is shown
together with the computed exponential regression
function f2(x) = 0.0000834*(0.954)x and our own
`halving function’ f3(x) = 0.000084*(1/2)x/hi where
the half-life hi is set using the sliding point H to a
value of about 14.7 seconds. The point I is taken on
the graph of f3(x) and the tangent is drawn. The ycoordinate of I is stored as it and the slope of the
tangent as sit. The value of the quotient p =sit/it is
calculated, and we see that as I slides on y = f3(x) this quotient p stays constant at -0.047. This is precisely
what is meant by an exponential model – the rate of change of the current is a constant multiple of the
current. The negative sign shows that the tangents are sloping downwards, so that the process is in decay.
𝑑𝑖
So the current function i(t) conforms to a differential equation of the form 𝑑𝑡 = 𝑝. 𝑖 – but we also need to
consider what a capacitor does. It stores electrical charge Q, measured in coulombs. The current i is the
rate of flow of charge. When the capacitor has been charged up, and the battery disconnected, the charge Q
stored in the capacitor diminishes, as the capacitor is discharged. So the relationship between i and Q is that
𝑑𝑄
i = − 𝑑𝑡 . The relationship between the charge Q held in the capacitor and the voltage difference V across it
is that Q/V = C, the capacitance. So we now have the theoretical elements from which to build the model!
𝑑𝑄
𝑑𝑄
𝑄
𝑑𝑉
𝑉
V = Q/C =iR = -R 𝑑𝑡 - so that 𝑑𝑡 = − 𝑅𝐶. Replacing Q by CV gives: 𝑑𝑡 = − 𝑅𝐶 .
Now some differential equations have simple solutions, while others can’t be solved exactly and need an
approximate technique. So we’ll start by illustrating a finite approximation technique invented by Euler. In
this spreadsheet we have the time t and voltage lists v from our experiment. The list pv evaluates our
halving model function f1(x) = v0*(1/2)-x/h with h = 14.65 seconds at each time in the list t. The list dv
evaluates the numerical derivative of f1(x)
at each time in the list t – and so gives the
slopes at each time. The list p holds the
quotients dv/dp. The values of r , c and
k = -1/rc are stored in column F together
with the time step dt = 0.1 secs. These are
used to generate the approximate Euler
solution av in column G using the formula
g2 = g1 + k*g1*dt with the initial voltage in
g1. The formula for g2 is then dragged
down as far as cell g181. As we can see the
numerical solution is slightly adrift from the
observed values – probably because the
values of either r or c or not exactly as
stated.
Finally we can see the connection
𝑑𝑉
𝑉
between the differential equation = −
𝑑𝑡
𝑅𝐶
and the number e.
The red graph is that of y = 2x, the green
graph is y = 3x and the blue one is y = nx. In
each case the tangent at (0,1) has been
drawn and the slope measured. By dragging
the slider n you can find an approximation to
the number e for which the tangent slope at
(0,1) is 1. The exponential function y = ex is a
fundamental tool in differential and integral
calculus since it has the property that:
𝑑𝑒 𝑥
𝑑𝑥
= 𝑒 𝑥 , and so:
𝑑𝑒 𝑘𝑥
𝑑𝑥
= 𝑘. 𝑒 𝑘𝑥 .
So now we have the theoretical solution to the RC-circuit problem. The voltage function is given by
V(t) = v0.e-t/RC – so that the derivative V’(t) = -1/RC*V(t). If we equate this to V(t) = v0.(1/2)t/h we just need to
solve e-t/RC = (1/2)t/h = 2-t/h and we solve this by taking logarithms of both sides to the base e (also known as
natural logs or Naperian logs) to give: -t/RC = -t/h.ln(2). Hence the half-life h is given theoretically by:
h = RC.ln(2). So finally for our assumed values of resistance R and capacitance C the theory predicts that the
half-life h of the voltage should be 22.ln2 = 15.25 seconds, compared with our observed value of 14.65 secs –
with a difference of about 4%.
King Arthur was the source of many legends, including that of `the
knights of the round table’. He is thought to have lived around 500AD.
The top of a huge wooden table 18 ft in diameter hangs on the wall of
Henry III’s Great Hall in Winchester. It weighs just about 1 ton and was
thought to be Arthur’s actual round table around which knights like Sir
Galahad and Sir Launcelot had sat. But how can we find out how old the
Winchester table really is?
In 1976 it was taken down for analysis by a team of archaeologists led
by Martin Biddle, Emeritus Professor at the University of Oxford. The
technique they used is now called radiocarbon dating and was carried
out at the UK Atomic Energy Laboratory at Harwell (now the
Rutherford-Appleton Laboratories). Here is the basic science. Carbon14, written 14C, is a radioactive isotope of carbon with a half-life of
5,730 years. A small amount of naturally occurring carbon in the
environment is 14C. Although 14C decays into nitrogen-14 through beta
decay, the amount of 14C in the environment remains constant because
new 14C is always being created in the upper atmosphere by cosmic
rays. Living things ingest materials that contain carbon, so the
percentage of 14C within living things is the same as the percentage of
14
C in the environment. Once an organism dies, it no longer ingests, so
the 14C in the organism is no longer replaced and the percentage of 14C
decreases as it decays.
By measuring the percentage of 14C in the remains of an
organism, and by assuming that the natural abundance of 14C has
remained constant over time, scientists can estimate when that
organism died. Let’s see if we can! Martin Biddle's team found that the percentage concentration of 14C in
the samples of wood taken from the Winchester round table was 91% so we should be able to create a
graph from which we can estimate the year in which the trees would have been cut.
So, just as with the voltage decay, we set up a half-life
model function f1(x). Its initial value will be 100 (for
100%) and the half-life h = 5730. We can set up a sliding
point D on the x-axis to control the date. Drawing a
perpendicular from D to the graph of y=f1(x), and then a
perpendicular from that intersection to the y-axis we can
read off the carbon-14 concentration (in %)
corresponding to the time D. We drag D until the
concentration shows 90.9%, and observe that it takes
about 789 years decay to reach this level. This would
date the wooden samples at having been cut down in
around 1187 AD – well into the Norman era.