Materials-Exam2
advertisement
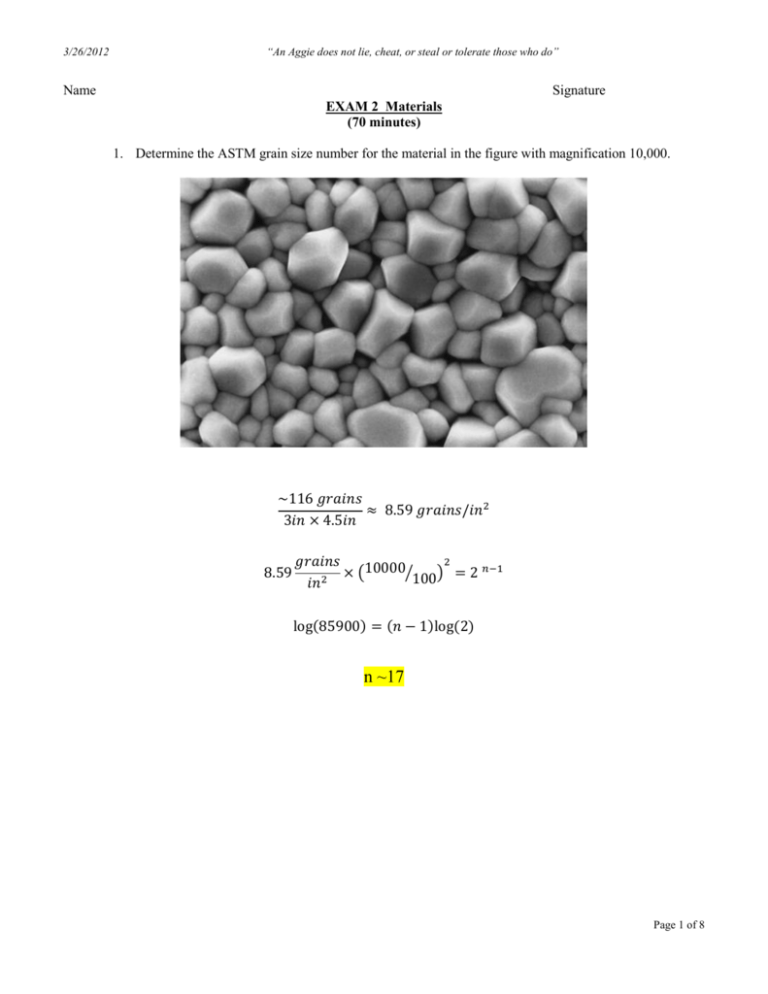
3/26/2012 “An Aggie does not lie, cheat, or steal or tolerate those who do” Name Signature EXAM 2 Materials (70 minutes) 1. Determine the ASTM grain size number for the material in the figure with magnification 10,000. ~116 𝑔𝑟𝑎𝑖𝑛𝑠 ≈ 8.59 𝑔𝑟𝑎𝑖𝑛𝑠/𝑖𝑛2 3𝑖𝑛 × 4.5𝑖𝑛 8.59 2 𝑔𝑟𝑎𝑖𝑛𝑠 𝑛−1 10000⁄ × ( ) 100 = 2 𝑖𝑛2 log(85900) = (𝑛 − 1)log(2) n ~17 Page 1 of 8 3/26/2012 “An Aggie does not lie, cheat, or steal or tolerate those who do” 2. What type(s) of bonds are found between atoms within hydrocarbon molecules? (A) ionic bonds (B) covalent bonds (C) van der Waals bonds (D) metallic bonds Page 2 of 8 “An Aggie does not lie, cheat, or steal or tolerate those who do” 3/26/2012 3. Assume there is one Schottky defect in every eight unit cells of ZnS, which has the zinc blende structure, with lattice parameter is 0.59583 nm. What is the density of the defective ceramic? The density of the ceramic In 8 unit cells, we expect 32 Zn + 32 S , but due to the defect: 32 Zn − 1= 31 32 S − 1= 31 𝜌= (31/32)(4)(65.41 𝑔/𝑚𝑜𝑙) + (31/32)(4)(32.06 𝑔/𝑚𝑜𝑙) = 2.97 𝑔/𝑐𝑚3 𝑎𝑡𝑜𝑚𝑠 (5.9583 ∗ 10− 8 𝑐𝑚)3 (6.02 ∗ 1023 𝑚𝑜𝑙 ) 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 = 𝑑𝑒𝑓𝑒𝑐𝑡𝑖𝑣𝑒 𝑐𝑒𝑙𝑙𝑠 𝑚𝑎𝑠𝑠 𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑐𝑒𝑙𝑙𝑠 Page 3 of 8 “An Aggie does not lie, cheat, or steal or tolerate those who do” 3/26/2012 4. Show that C x = x2 C 2C B is a solution to = D 2 The parameter B is independent exp t x . Dt 4Dt of both x and t. Calculating x2 x2 C B = 1 exp t 2D1/ 2t 3 / 2 2Dt 4 Dt And D 2C B = 2 1/ 2 3 / 2 x 2D t x2 x2 1 exp 2 Dt 4 Dt Both yield the same results Page 4 of 8 “An Aggie does not lie, cheat, or steal or tolerate those who do” 3/26/2012 5. Iron containing 0.05% C is heated to 912oC in an atmosphere that produces 1.20% C at the surface and is held for 24 h. Calculate the ratio of carbon content (CxBCC/CxFCC) at 0.05 cm beneath the surface. Where CxBCC is the Cx when iron is in a BCC phase and CxFCC for iron in a FCC phase. T= (24 h)(3600 s/h) = 86,400 s 𝐷𝐵𝐶𝐶 = 6.2 × 10 𝐷𝐹𝐶𝐶 = 2.3 × 10 𝐵𝐶𝐶: −7 −5 𝑚 2⁄ −80000 𝑠 exp ( 8.314 𝑚 1.2 − 𝐶𝑥 = erf 1.2 − 0.05 2⁄ 𝐽 𝑚𝑜𝑙 ) = 1.845 × 10−10 𝑚2 /𝑠 𝐽 𝑚𝑜𝑙 ) = 6.883 × 10−12 𝑚2 /𝑠 𝐽 × 1185 𝐾 𝑚𝑜𝑙 𝐾 −148000 𝑠 exp ( 8.314 𝐽 × 1185 𝐾 𝑚𝑜𝑙 𝐾 0.0005 𝑚 √ [2 1.845 × 10−10 𝑚2 × 86,400 𝑠] 𝑠 = erf[0.0626] = 0.0705 CxBCC : 1.12% 𝐹𝐶𝐶: 1.2 − 𝐶𝑥 = erf 1.2 − 0.05 0.0005 𝑚 2 −12 𝑚 × 86,400 𝑠 √ [2 6.883 × 10 ] 𝑠 = erf[0.3242] = 0.3532 CxFCC : 0.79% CxBCC/CxFCC = 1.41 Page 5 of 8 3/26/2012 “An Aggie does not lie, cheat, or steal or tolerate those who do” 6. Calculate the molecular weight of a 5000-mers copolymer produced by 1 kg of ethylene and 3 kg of propylene. Page 6 of 8 “An Aggie does not lie, cheat, or steal or tolerate those who do” 3/26/2012 7. The following data (plotted) were collected from a 20 mm diameter test specimen of a ductile cast iron (l0 = 40.00 mm); after fracture, the gage length is 47.42 mm and the diameter is 18.35 mm. Solution Calculate (a) the 0.2% offset yield strength, (b) the tensile strength, (c) the modulus of elasticity, (d) the %Elongation, (e) the %Reduction in area, (f) the engineering stress at fracture, (g) the true stress at fracture (h) the modulus of resilience. ½(yield strength)2(modulus of elasticity)= ½ (240MPa)2(172000MPa) = 0.17 MPa Page 7 of 8 “An Aggie does not lie, cheat, or steal or tolerate those who do” 3/26/2012 8. The net bonding energy EN between two isolated positive and negative ions is a function of A B interionic distance r as follows: E N m n , where A, B, m, and n are constants for the r r particular ion pair. This equation is also valid for the bonding energy between adjacent ions in solid materials. The modulus of elasticity E at the equilibrium interionic separation ro is, dF E L dr ro . Derive an expression for the dependence of the modulus of elasticity on these A, B, m, and n parameters (for the two-ion system). 𝐹= 𝑑𝐸𝑁 𝑑𝑟 𝑑𝐸𝑁 = 𝐴𝑚𝑟0 −(𝑚+1) − 𝐵𝑛𝑟0 −(𝑛+1) 𝑑𝑟 𝑑𝐸𝑁 =0 𝑑𝑟 1 𝑦𝑖𝑒𝑙𝑑𝑠 → 𝐴𝑚 𝑚−𝑛 𝑟0 = ( ) 𝐵𝑛 Solution −(𝑚+2) 𝑚−𝑛 𝐴𝑚 dF 𝐸 = 𝐿 ) = L (−𝐴𝑚(𝑚 + 1) ( 𝐵𝑛 dr ro −(𝑛+2) 𝑚−𝑛 𝐴𝑚 + 𝐵𝑛(𝑛 + 1) ( ) 𝐵𝑛 ) Page 8 of 8