Percent solution guide - Bio-Link
advertisement

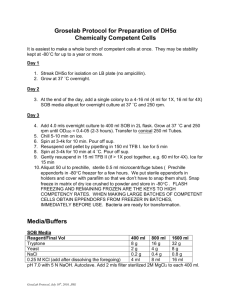
1 SOLUTIONS EXPRESSED AS PERCENTS Study guide and self-test Solution concentrations in the biotechnology laboratory are often expressed as percent solutions in one of three ways % w/v = 1 gram solute % v/v = 1 mL solute % w/w = 1 gram solute 100 mL solution 100 mL solution 100 g solution Let’s try a few examples: 1. Calculate how to make 300 mL of a 35% v/v solution of ethanol in water 2. Calculate how to make 5 L of a 0.2% v/v solution of methanol in water 3. Calculate how to make 1 kg of a 5% w/w solution of NaCl in water 4. Calculate how to make 250 mL of a 4.3% w/v solution of KCl in water 5. Calculate how to make 1.3 L of a 1.4% w/v solution of MgCl2 in water 2 ANSWERS: 1. Calculate how to make 300 mL of a 35% v/v solution of ethanol in water 35% = 35 mL ethanol 100 mL solution ___y___ 300 mL solution = 35 mL ethanol 100 mL solution y = (35 mL ethanol)(300 mL solution) = 105 mL ethanol 100 mL solution Measure out 105 mL ethanol and bring to a volume (BTV) of 300 mL with water. 2. Calculate how to make 5 L of a 0.2% v/v solution of methanol in water 0.2% = 0.2 mL methanol 5 L solution = 5000 mL 100 mL solution ___y___ 5000 mL solution = 0.2 mL methanol 100 mL solution y = (0.2 mL methanol)(5000 mL solution) = 10 mL methanol 100 mL solution Measure out 10 mL methanol and bring to a volume (BTV) of 5 L with water. 3 3. Calculate how to make 1 kg of a 5% w/w solution of NaCl in water 5% = 5 g NaCl 1 kg solution = 1000 g solution 100 g solution ___y___ 1000 g solution = 5 g NaCl 100 g solution y = (5 g NaCl)(1000 g solution) = 50 g NaCl 100 g solution Measure out 50 g NaCl and add 950 g water. 4. Calculate how to make 250 mL of a 4.3% w/v solution of KCl in water 4.3% = 4.3 g KCl 100 mL solution ___y___ 250 mL solution = 4.3 g KCl 100 mL solution y = (4.3 g KCl)(250 mL solution) = 10.75 g KCl 100 mL solution Measure out 10.75 g KCl and bring to a volume (BTV) of 250 mL with water. 5. Calculate how to make 1.3 L of a 1.4% w/v solution of MgCl2 in water 1.4% = 1.4 g MgCl2 1.3 L solution = 1300 mL 100 mL solution ___y___ 1300 mL solution = 1.4 g MgCl2 100 mL solution y = (1.4 g MgCl2)( 1300 mL solution) = 18.2 g MgCl2 100 mL solution 4 Measure out 18.2 g MgCl2 and bring to a volume (BTV) of 1.3 L with water.