revised background research - aos-hci-2012-research
advertisement

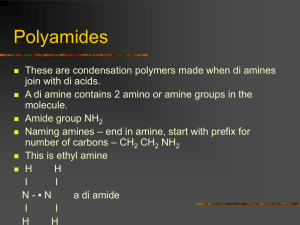
Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 Testing salt induced spider-net linkage in electrospun nanowebs of poly(vinyl alcohol) and poly(ethylene-co-vinyl alcohol) Applications for nonwoven membranes, which can be spun out of synthetic and natural polymers, are extremely varied. They include filtration, insulation, bone and tissue scaffolding, and wound dressing. Electrospun membranes are suited to these applications because of their high surface to volume ratios and their interconnecting porous networks; however, they can often be very fragile (Bhardwaj & Kundu, 2010; Yang, Li, & Nie, 2007). Electrospinning is a process for creating non-woven membranes (Wong, Hutter, Zinke-Allman, & Wang, 2009). A polymer solution, such as poly(vinyl alcohol) (PVA) is placed in a capillary needle. When a voltage is induced between the needle and a collector plate, a surface charge is induced in the solution causing a spherical drop on the end of the needle to deform into a conical shape, called a “Taylor Cone”. After a critical voltage is reached, the electrostatic repulsion force of the surface charges overcomes the surface tension of the solution and the charged fluid is released from the tip of the Taylor Cone. High surface charge densities enhance a whipping mode, where bending of the jet creates highly stretched fiber as well as rapid evaporation of the solvent. The fibers are deposited on the collector in the form of non-woven mat. Individual fiber diameters are usually between 100 nanometers and 1 micrometer (Bhardwaj & Kundu, 2010; Yang et al., 2007). PVA is a semi-crystalline, hydrophilic polymer with fairly good chemical and thermal stability. It is water soluble with a high permeability rate as well as being very biocompatible (Koski, Yim, & Shivkumar, 2003). PVA also has good chemical resistance and excellent mechanical properties (Nirmala et al., 2011) and is synthesized from poly(vinyl acetate) through hydrolysis and can be broken into two groups, partially hydrolyzed and fully hydrolyzed. PVA is chemically stable at normal temperatures and its physical and chemical properties have led it 1 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 to be used in film, contact lenses, and biomedical applications (Zhang, Yuan, Wu, Han, & Sheng, 2004). Poly(ethylene-co-vinyl alcohol) (EVOH) is hydrolyzed from poly(ethylene-co-vinyl actetate), and is most commonly available in 55-70 mol% vinyl alcohol content (Kenawy et al., 2003; Marias et al., 2006). The gas barrier properties exhibited by EVOH and its resistance to chemicals make it a useful material in food packaging, especially as an oxygen barrier (Marias et al., 2006). When intended to be used with liquid or high humidity, EVOH needs to be laminated with a hydrophobic material such as polyethylene to preserve its ability as an oxygen barrier, which is greatly compromised by the absorption of water (Milosavljevic & Thomas, 2006). In a study done by Kenawy et al. (2003), EVOH polymers were electrospun in solutions with a solvent of 70/30% isopropanol/water, with compositions of 2.5-20% w/v. The ideal time for mixing these solutions was found to be about 2-3 hours, and the ideal temperature about 80° C. The solutions were cooled to room temperature, approximately 22-24° C, before electrospinning. The polymer began to precipitate after a few hours, so solutions were electrospun within 2-3 hours after mixing them. If not, a precipitated mixture could be solubilized again at 50°C for 10 minutes. The electrospinning set-up consisted of a blunt-end needle and a syringe, which was filled with solution and placed about 20 cm from the target surface. The flow rate of the solution was 10-18 mL/h. For 62 mol% vinyl alcohol, spinning started at 5% w/v solution, and the most uniform fibers were obtained at 10% w/v (Kenawy et al., 2003). We plan to use these parameters when spinning our EVOH and alter them to fit our specific procedures. Non-woven membranes can be affected by many different factors including concentration, conductivity, and viscosity of the solution, diameter of the syringe needle, 2 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 distance between the needle and the collection plate, and electric voltage (Yang et al., 2007). Changing the viscosity of the solution has been found to affect the length and diameter of the fibers, by changing how quickly the solution is released from the Taylor Cone (Huang, Zhang, Kotaki, & Ramakrishna, 2003). High molecular weight polymer solutions are usually used because they provide an optimum viscosity for electrospinning (Bhardwaj & Kundu, 2010). Although a higher voltage can generate finer nanofibers and decrease the diameter of beads, it does not reduce the number of beads formed. Increasing the voltage has actually been found to cause more beaded and uneven fibers (Huang et al., 2003). Increased beading can also be caused by having too high of a flow rate of solution from the syringe (Bhardwaj & Kundu, 2010). Another way to change the membranes is by adding a filler, such as a salt, to the polymer solution. Fillers, including both organic and inorganic substances, have been found to greatly reduce or eliminate the presence of beads in the nanowebs (Huang et al., 2003). It has been found that by adding a filler, such as a salt, into the polymer solution, the mechanical properties can be improved. With an addition of 1 wt% salt to a PLDA polymer the fibers were thinner and completely free of beads (Huang et al., 2003). In a study performed by Wu, Fan, Qin, and Zhang (2007) it was found that the addition of TiO2 to PVA resulted in a membrane with better thermal radiative properties, because the average fiber diameter had decreased. The addition of a salt, such as NaCl or KBr, in Nylon 6 (N6) or PVA, has been found to create spider-net links between main fibers, linking them together (Barakat, Kanjwal, Sheikh, & Kim, 2009). The spider net links have been found to alter mechanical strength, surface morphology, thermal properties, wettability, ultraviolet light blocking abilities, and antimicrobial properties. In fact, with the addition of TiO2 to N6, it was found that the maximum thermal degradation was shifted 2.6 ˚C higher, mechanical strength was increased, strain was reduced, 3 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 hydrophilicity was increased, and fouling was prevented (Pant et al., 2010). The increased strength and other changes caused by the addition of salts could be very useful in applications such as protective clothing In order to create this linkage, the optimum amount of salt is about 1.5 wt%. The spidernet link formations are believed to be created by free ions, resulting from the dissociation of the ionic salts, such as that caused by placing them in water (Huang et al., 2003). It has also been found that most of the salt in the solution was found in the spider-net links. It was discovered in a recent study, that higher concentrations of salt such as 5 wt% or 10 wt% caused little to no spider-net link formation and unequal distribution of the salt throughout the web (Pant et al., 2010). In a study conducted by Barakat et al. (2009), it was determined that when the salts were mixed with the polymer solutions for longer amounts of time, the resulting spider-net links were more present and developed. The best length of stirring was determined to be about 24 hours, and these spider-link webs were shown to have greater structural stability and resistance to strain. However, if the solution was not mixed for long enough, the spider-net link formation was inconsistent. It was proposed, in this experiment, that the formation of webs would be similar in all polymer solutions that have a polar base. Our experiment will be similar to this one; however, instead of testing the effects of salts on N6 and PVA, we will be testing them on PVA and EVOH, both of which have water, a polar liquid, as a solvent. Our goal is to determine if, like hypothesized by Barakat et al. (2009), the web formations are created in other solutions with polar solvents. To test the effect of adding a salt to the polymer solution, we plan to test mechanical properties. Mechanical properties such as strain softening behavior, extensibility, maximum stress, energy loss, and deformation characteristics can be measured. Stress is the force per unit 4 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 area applied to the material and strain is how much the material changes its shape; in this case, it would be how much the nanofiber bends from the straight position. Extensibility of a fiber is the strain at which that fiber snaps, and maximum or peak stress is the highest stress value reached before the fiber snaps. Deformation measures how closely a fiber returns to its original shape after stretching (Carlisle, Coulais, & Guthold, 2010). Surface morphology can also be observed using scanning electron microscopy (SEM), with which the presence of spider-net links can be determined (Barakat et al., 2009). In review, our project will be to test spider-net link formation in two different polymers, PVA and EVOH, both of which are biocompatible. The addition of spider-net links has been found to increase the mechanical strength of the electrospun mat, making it more attractive for applications such as protective clothing (Barakat et al., 2009; Pant et al., 2010). We will attempt to induce spider-net links by adding 1.5 wt% salt to the polymers, and we will be careful to avoid using more than this amount so that we do not have uneven spider-net link formation. The addition of the salts should also help to reduce beading (Barakat et al., 2009; Huang et al., 2003). Our project will differ from other research that has been done, because we plan on using other salts than those previously tested, and we will be testing the formation of these spider-net links on EVOH. 5 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 References Barakat, N. A. M., Kanjwal, M. A., Sheikh, F. A., & Kim, Y. H. (2009). Spider-net within the N6, PVA and PU electrospun nanofiber mats using salt addition: Novel strategy in the electrospinning process. Polymer, 50, 4389-4396. doi: 10.1016/j.polymer.2009.07.005 Bhardwaj, N., & Kundu, S. C. (2010). Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances, 28, 325-347. doi: 10.1016/j.biotechadv.2010.01.004 Carlisle, C. R., Coulais, C., & Guthold, M. (2010). The mechanical stress-strain properties of single electrospun collagen type I nanofibers. Acta Biomateriala, 6, 2997-3003. doi:10.1016/j.actbio.2010.02.050 Grundke, K., Michel, S. Knispel, G., & Grundler, A. (2008). Wettabality of silicone and polyether impression materials: Characterization by surface tension and contact angle measurements. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 317, 598-609. doi: 10.1016/j.colsurfa.2007.11.046 Huang, Z.-M., Zhang, Y.-Z., Kotaki, M., & Ramakrishna, S. (2003). A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compostites Science and Technology, 63, 2223-2253. doi: 10.1016/S0266-3538(03)00178-7 Kenawy, E.-R., Layman, J. M., Watkins, J. R., Bowlin, G. L., Matthews, J. A., Simpson, D. G., & Wnek, G. E. (2003). Electrospinning of poly(ethylene-co-vinyl alcohol) fibers. Biomaterials, 24, 907-913. doi: 10.1016/S0142-9612(02)00422-2 6 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 Koski, A., Yim, K., & Shivkumar, S. (2003). Effect of molecular weight on fibrous PVA produced by electrospinning. Materials Letters, 58, 493-497. doi: 10.1016/S0167577X(03)00532-9 Marias, S., Hirata, Y., Cabot, C., Morin-Grognet, S., Garda, M.-R., Atmani, H., & PoncinEpaillard, F. (2006). Effect of a low-pressure plasma treatment on water vapor diffusivity and permeability of poly(ethylene-co-vinyl alcohol) and polyethylene films. Surface and Coatings Technology, 201, 868-879. doi:10.1016/j.surfcoat.2005.12.049 Milosavljevic, B. H., & Thomas, J. K. (2006). Radiation induced processes in the co-polymer, polyethylene–poly(vinyl alcohol). Nuclear Instruments and Methods in Physics Research B, 201, 185-190. doi:10.1016/S0168-583X(03)00628-1 Nirmala, R., Kalpana, D., Jeong, J. W., Oh, H. J., Lee, J.-H., Navamathavan, R., … Kim, H. Y. (2011). Multifunctional baicalein blended poly(vinyl alcohol) composite nanofibers via electrospinning. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 384, 605-611. doi: 10.1016/j.colsurfa.2011.05.009 Pant, H. R., Bajgai, M. P., Nam, K. T., Seo, Y. A., Pandeya, D. R., Hong, S. T., & Kim, H. Y. (2010). Electrospun nylon-6 spider-net like nanofiber mat containing TiO2 nanoparticles: A multifunctional nanocomposite textile material. Journal of Hazardous Materials, 185, 124-130. doi: 10.1016/j.jazmat.2010.09.006 Wong, K. K. H., Hutter, J. L., Zinke-Allman, M., & Wang, W. (2009). Physical properties of ion beam treated electrospun poly(vinyl alcohol) nanofibers. European Polymer Journal, 45, 1349-1358. doi: 10.1016/j.eurpolymj.2009.02.002 7 Names: Lindsey Vanderlyn and Lamiya Zavari Date Due: 09/19/2011 Wu, H., Fan, J., Qin, X., & Zhang, G. (2007). Thermal radiative properties of electrospun superfine fibrous PVA films. Materials Letters, 63, 828-831. doi: 10.1016/j.matlet.2007.06.080 Yang, D., Li, Y., & Nie, J. (2007). Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydrate Polymers, 69, 538-543. doi: 10.1016/j.carbpol.2007.01.008 Zhang, C., Yuan, X., Wu, L., Han, Y., & Sheng, J. (2004). Study on morphology of electrospun poly(vinyl alcohol) mats. European Polymer Journal, 41, 423-432. doi: 10.1016/j.eurpolymj.2004.10.027 8