Lecture 28

Physics 249 Lecture 28, Nov 9th 2012
Reading: Chapter 9
HW 8 due today. No homework next week. Exam Wednesday Nov 14 th .
Exam comments. The exam will cover section 6.6 (transition and reflection of waves),
Chapter 7, Chapter 9 through molecular spectrum, and related material from the HW and lecture. The exam will again be a mix of challenging questions, interpretation of the answers to those questions, and simpler computation questions.
1) Revisiting the black body spectra.
As a precursor to molecular spectra lets revisit the black body distribution.
The black body distribution was a continuous distribution describing the energy density as a differential function of wavelength. The form of the distribution could be derived by assuming that at any given wavelength light was emitted in quantized energy packets photons. The frequency and energy of a photon was directly related to the wavelength.
A higher energy density corresponded to emitting more quantized photons rather than light with the same wavelength but greater amplitude.
For an approximately perfect black body to exist there had to be some quantum system that has a high enough density of energy states, and thus density of possible energy transitions, to be able to emit or absorb an approximately continuous spectrum. The atom is certainly not this quantum system since the energy levels are quite discrete. Especially transitions between the lower energy levels are observed as discrete lines.
To understand this problem lets study the energy levels of the molecule.
2) Molecular transition.
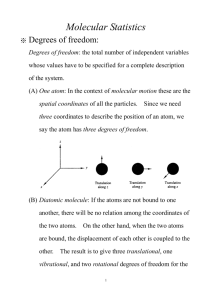
There are three types of molecular transitions. a) Atomic transitions involving the atoms of the molecule. These occur at eV level energies though energies can be as high as keV. b) Transitions between oscillatory levels. The potential for a molecular bond whether covalent of ionic is approximately parabolic like a harmonic oscillator potential and oscillatory excited states are possible. These occur at the level of ~0.1 eV. c) Transition between rotational levels. This ystem can have rotational quantum energy levels as well as the vibrational energy levels discussed above. These occur at the level of ~0.0001 eV.
Note that the potential of the system is not similar to the coulomb potential and vibrational energies and rotational energies are order of magnitude different from each other. The atomic treatment solved the full Schrodinger equation in all three dimensions.
In this case it makes sense to separate vibrational and rotational energy mode treatments.
The combination of the three sets of scales each with many energy modes allows the approximately continuous spectrum on photon transitions suggested by the black body result.
3) Atomic transitions.
Atomic transitions can have a substantial range.
Hydrogen atom transition can range from 13.6eV to dissociate a ground state electron to very small transitions between adjacent levels with high n.
General ionization energies can range from 25eV in Helium to 3eV for the outer, valence, electrons in large atoms.
Xray energies in transitions to an inner shell of a large atom can be as large as several keV.
4) Vibrational energy levels
The potential of ironically or covalently bonded atoms is approximately the same as the parabolic shape of the harmonic oscillator. Therefore you would expect approximately equally spaced energies levels with energies
𝐸 = (𝑛 +
1
2
) ℏ𝜔
We can understand the angular frequency in terms of the characteristic size of the potential well by an analysis based on the uncertainty principle. Recall from lecture 14 we found that the minimum energy and corresponding minimum uncertainty occurred ℏ when ∆𝑥 = √
2𝑚𝜔
. ∆𝑥 is representative of the average size of the potential well, r
0
.
Therefore:
𝐸 = (𝑛 +
1
2
) ℏ 2
2𝑚𝑟
0
2
Note that this is different, and more exact, than the books treatment that involves approximating the harmonic oscillator as a square well.
Also remember that there are selection rules related to considering whether there is effective overlap between the wave functions in a transition. There rules only allow transitions between adjacent n levels. This rule can be broken when transitioning between different atomic energy levels and molecular vibrational levels simultaneously.
Changing atomic levels changes the molecule, for instance altering the separation distance, and the energy structure of the vibrational levels. This will create overlap
between the wave functions of more widely spaced vibrational levels and allow transitions.
Typical transition energies are on order 0.1eV.
6) Rotational energy levels.
Consider the classical energy of a rotating system.
𝐸 𝑟𝑜𝑡
=
1
2
𝐼𝜔 2 =
1
2
(𝐼𝜔)
𝐼
2
=
𝐿 2
2𝐼
We understand how to quantize this. 𝐿 2
is defined by the angular part of the kinetic energy in the wave equation and I which involves the masses and radii does not change.
𝐸 𝑟𝑜𝑡 𝜓 =
𝐿 2
2𝐼 𝜓 = 𝑙(𝑙 + 1)ℏ
2𝐼
2 𝜓
Transitions between levels are constrained by the selection run that Δ𝑙 = ±1 . Thus an arbitrary transition can be understood from
[(𝑙 + 1)(𝑙 + 2) − 𝑙(𝑙 + 1)]ℏ 2
Δ𝐸 𝑙,𝑙+1
=
2𝐼
=
(𝑙 + 1)ℏ 2
𝐼 and
𝐼 = 𝑚
1 𝑟
1
2 + 𝑚
2 𝑟 2
2
= 𝐼𝜇𝑟
0
2 where 𝑟
0
= 𝑟
1
+ 𝑟
2
and 𝜇 = 𝑚
1 𝑚
2 𝑚
1
+𝑚
2
Typical rotational energies are about 1x10
-4
to 1x10
-3
or as big as 1x10
-2
eV for H2.
Using the equations above you can calculate the separation distances between atoms in molecular bonds. This is how they are typically determined. Note that the covalent bonds are typically much smaller than the ionic bonds.
The rotational frequencies are orders of magnitude smaller than the vibrational energy modes which validates the idea of treating the separately.
The rotational modes energies are of the same magnitude as thermal kinetic energy in a gas.
Note that you can get rotational energies with large l that reach vibrational levels.
7) Molecular energy level. The large number of energies levels and the order of magnitude differences in energy levels for the different types of transitions lead to a densely filled energy spectrum.
Also there are a large number of processes via which and molecule can interact. These processes can involve photons with energies exactly consistent transition energies or the cases of Compton effect, (inverse) photo effect and Rayleigh scattering involve arbitrary transitions.
Note we already discussed absorption, emission and stimulated emission in lecture 3.