Cell respiration reading and Questions
advertisement

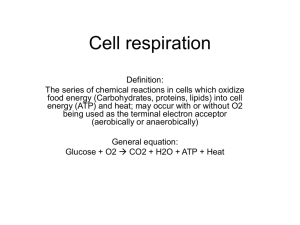
Cell Respiration: Getting the energy back out of glucose. Now that we have an understanding of how autotrophs lock energy away in glucose, we need to take a look at how all organisms extract that energy in order to transfer it to ATP, the only energy molecule our cells can actually use. First let's refresh some basics. The process by which cells chemically transfer the energy from glucose to ATP is called cellular respiration. Every single living thing on the planet performs cell respiration in one way or another. Even the plants! It might seem silly to go through all of photosynthesis building glucose if we are just going to break it back down, but believe it or not, we can get more ATP by doing both processes than we can by doing just one. Plus it allows plants to store glucose for a rainy day, something you, too, should be thankful for. Like photosynthesis, cell respiration has a summary reaction: what goes in, what comes out and where does the energy go. Figure 1: Summary Reaction for Cell Respiration There are, of course, many more molecules, enzymes, and cell structures involved, and it takes MANY steps. Once again, the majority of these molecules are recycled and returned to their original state by the end of the process so there is no need to list them in the summary. When you look at the summary reaction shown in figure 1, you should recognize two things: 1. It looks an awful lot like the photosynthesis reaction in reverse. - This is not a coincidence. Cell respiration is very much like photosynthesis in reverse. The products of one are the reactants of the other. Photosynthesis absorbs light energy to store in glucose; cell respiration extracts that energy from glucose. As we go through the process we’ll see more examples of how cell respiration is sort of “undoing” what photosynthesis did. 2. It looks like a type of combustion reaction - Again, not a coincidence. Cell respiration is a controlled combustion reaction. The same type of reaction that happens when we burn logs in a fireplace or burn gasoline in a car. In all three cases you are combining an organic compound (wood, gasoline or glucose) with oxygen from the atmosphere and converting it into carbon dioxide and water. In a combustion reaction all the potential energy in the organic compound is transformed (heat/light from the fire, energy to move the car from the gasoline, ATP from glucose). The difference is that we don’t want living things to burst into flames with a sudden release of energy like when gasoline or wood is burned. For this reason cell respiration is very controlled and releases small bits of energy a little bit at a time. The many reactions of cell respiration make this possible. Let’s dive into the process. We are going to break the process down into steps, but don’t lose sight of the big picture. Like we saw with photosynthesis, there are a lot of details and there is a lot of chemistry, but along the way we’ll be pulling out the big ideas on which you should focus. Glycolysis: splitting the glucose No matter what species you are, the very first step of extracting the energy from glucose is glycolysis. As the name literally means, the purpose of glycolysis is to split a glucose molecule into two 3-carbon molecules called pyruvate. While it takes ten different chemical reactions to do this (along with 10 enzymes and various energy molecules) it doesn’t require any specialized cell structures. All of the molecules needed for glycolysis are present right in the cytoplasm of all metabolically active cells in all species. It doesn’t matter whether the organisms is single celled or billions of cells. If it is performing cell respiration it must begin with glycolysis. (see figure 2) Figure 2: Glycolysis Figure 2 summarizes the 10 steps of glycolysis into the important ideas. We start with glucose. In the beginning steps we must invest a little bit of energy to get the ball rolling. We can see that 2ATP are “used” in these steps meaning that they are being converted into 2ADP and that the energy from those 2ATP is being transferred into the organic compounds being created along the way. Once we have invested a little bit of energy we split the glucose into two 3C molecules. From here out, the reactions are all about harvesting energy from those two 3C molecules. By the time we have turned each of these 3C molecules into pyruvate we will have phosphorylated four ADP to make ATP and transferred electrons into 2 NAD+ to make 2NADH. So in terms of the organic compounds we’ve done the following: C6H12O6 2C3 H4O3 (the “missing” H’s were transferred to the electron carriers or released) In terms of energy molecules we have this: (-2ATP) + 4ATP + 2NADH = NET GAIN of 2ATP + 2NADH This might not be terribly impressive but keep in mind we’ve techinically only broken one carbon bond. There are still many high energy bonds in our 2 pyruvates from which we can extract more energy. Figure 3: Aerobic vs Anaerobic Decision The Pyruvate Decision: To respire aerobically, or not to respire aerobically, that is the question. (Shakespeare had it all wrong…) Once we have completed glycolysis and have our 2 pyruvate molecules, the process comes to a fork in the road and cells must make an important decision about how to proceed. The direction they choose will be dependent on both the components of their cells as well as the environment in which they are respiring. The options are to proceed with aerobic respiration or anaerobic respiration. Continuing aerobically will gain the cell many more ATP, but it requires having access to both mitochondria and oxygen. The anaerobic option doesn’t require oxygen nor does it require mitochondria, but it means getting a lot less energy per glucose. In reality this isn’t really a “choice”. Obviously if you don’t have mitochondria, you have to go the anaerobic route. Conversely, cells with access to both mitochondria and oxygen will always take the aerobic path since it will result in a greater ATP yield. We are going to explore the aerobic pathway first. Continuing down the Aerobic Pathway. Assuming a cell has mitochondria and oxygen it will always “choose” this path. This path involved three more steps: pyruvate oxidation, the Krebs cycle and the Electron transport Chain. By the end we’ll have broken down the rest of our carbon compounds and generated a whole bunch of ATP. 1. Pyruvate Oxidation In order to proceed aerobically we must first modify pyruvate in preparation for the krebs cycle. First we need to shuttle pyruvate from the cytoplasm into the mitochondria. Once there, an enzyme will remove CO2 from pyruvate and attach a “helper molecule” called Coenzyme A. In doing this, some electrons are transferred to NAD+ to make NADH, another energy molecule (add this to our tally!) The CO2 that was removed will just be released as a waste product. This part of the CO2 we see in the products of the summary reaction. The remaining 2 carbons from pyruvate attached to the coenzyme A form a molecule called acetyl CoA, and is the molecule that will be fed into the Krebs cycle. Each pyruvate we made during glycolysis will form one acetyl-CoA. 2. The Krebs Cycle- break down the rest of the carbons; fill electron carriers Figure 4 Krebs cycle The diagram above shows the pyruvate oxidation as well as the Krebs cycle so we can see how they are connected, but we will begin our description with Acetyl-CoA (look for the star). The Krebs cycle behaves in many ways like the Calvin Cycle we discussed in photosynthesis. There are starting molecules, we are adding to those molecules, a number of changes will happen to those molecules, and, in the end, we will be back where we started. This time the molecule being added to the cycle is what was left over after the pyruvate oxidation. The CoA will be removed from the Acetyl-CoA and recycled while the 2 carbons will be attached to a molecule in the cycle to make citric acid. You may see the Krebs cycle referred to as the Citric Acid cycle because it is the first molecule generated in this process. The rest of the cycle has two goals – a. Break the rest of the high energy bonds and transfer the energy to our energy molecules o Notice as you go around the cycle you see CO2 coming out. Each of those CO2 molecules is coming from the Acetyl-CoA that came into the cycle, which means they were part of the pyruvates you o made during glycolysis, which means they were once part of the glucose you ate! All of the CO2 molecules will become waste products that get exhaled. Also you should notice that 3NADH, 1FADH2 and 1ATP are also made in this process. The energy/electrons to make these all came from the bonds of our carbon molecules. (Make sure to add them to our tally! By the way, how many acetyl-CoA’s did we get from our glucose?) b. Regenerate the starting molecule for the Krebs cycle (called OAA). o The final steps are just about regenerating oxaloacetate (OAA) so that the Krebs cycle is ready to go again the next time an Acetyl CoA shows up. 3. The electron transport chain – convert energy in electron carriers to ATP Figure 5: Electron transport chain of the Mitochondrion By the time the Krebs cycle is done, we have no more carbons to work with; they have all been exhaled as CO2. All we have left are a few ATP and a big pile of electron carriers. The ATP is what we want, but we need to do something about those electron carriers. If only there were some way to transfer the energy from the electron carriers to ATP. Oh wait!! THERE IS! (Gosh, they’ve thought of everything….) Embedded in the membranes of the mitochondria are a number of molecules that make up an electron transport chain. When the electron carriers arrive, they donate their electrons to the first member of the chain. From here, the electrons go bouncing from molecule to molecule down the chain. As they do so they lose energy. This energy is used to pump H ions across the membrane from low to high concentration to establish a concentration gradient. WAIT A MINUTE! This is starting to sound awfully familiar. This is EXACTLY what happened during our light reactions of photosynthesis. Yes, the source of the electrons is different but the process is identical. So once we have a concentration gradient of H ions, can you guess what happens next? You got it! There also happens to be ATP synthase stuck in the mitochondrial membrane. The hydrogens flow through this enzyme allowing it to add the third phosphate to ADP to make ATP. But where do the electrons end up? Waiting at the end of the electron transport chain is little ol’ Oxygen. The same oxygen you inhaled during your last breath. By the time the electrons have finished bouncing down the chain, they are rather low in energy. Oxygen grabs on to them, along with a couple hydrogen ions, and creates water. THAT is the ONLY reason you breathe. You breathe so that oxygen can make its way to the mitochondria to pick up electrons at the end of the electron transport chain. It may seem silly, but it is crucial to your survival that cell respiration continue and oxygen is necessary to do this. Every electron carrier that we’ve made since the very beginning will donate their electrons to this chain ultimately resulting in the generation of ATP. In fact, when all is said and done, the ETC will generate roughly 32 ATP from the energy in all of our electron carriers. These 32 ATP in addition to the others we have made throughout the cell respiration process can now be used by the cell. The Anaerobic Pathway What we just read is great for all those organisms whose cells have mitochondria and live in well oxygenated environments. But, for cells that don’t, there needs to be another approach. Anaerobic respiration is the only other option. Let’s go back to the beginning. As we said, all cells begin with glycolysis. All cells split glucose into two pyruvate molecules and, in doing so, have a net gain of 2ATP and 2NADH. This is where the cells that lack mitochondria hit a snag. Without mitochondria we can’t proceed with pyruvate oxidation or the krebs or the ETC. The enzymes for these reactions are just not present in the cytoplasm. That means, not only can we not breakdown our pyruvate any further, but we also can’t empty the NADH we generated during glycolysis. This might not seem terribly tragic at first, but remember we have a limited number of electron carriers to fill. If they don’t get emptied the chemical reactions that happen during glycolysis that normally fill them will come to a grinding halt. No glycolysis, no ATP. That is a HUGE problem. For this reason, what follows glycolysis in the absence of mitochondria/oxygen focuses solely on emptying those electron carriers so that future rounds of glycolysis may happen. This process is called Fermentation. There are many types of fermentation depending on the waste products that are generated, but they all have one, and only one, goal: turn NADH back into NAD+. No additional ATP is generated in fermentation. An organism choosing this route must survive on the ATP generated during glycolysis ONLY. Discussion questions: 1. Complete this table to keep a tally of what is being made/used and during what phase. Which (if any) reactants from the summary reaction are used? Which (if any) products from the summary reaction are made? Where do these reactions take place? How many ATP are generated? (net) How many electron carriers are generated? Glycolysis Pyruvate oxidation Krebs cycle ETC 2. Using the summary reactions for photosynthesis and cell respiration, draw a diagram showing how they form a cycle. Indicate where energy is entering and leaving this cycle AND the form that energy is in at that point in time. 3. Why is it essential to plants that they do BOTH photosynthesis and cell respiration? 4. If Cell respiration were a single chemical reaction that released all the energy at once, what would happen to our cells? Glycolysis: 1. What does the fact that glycolysis is found in ALL living things suggest about its evolutionary history? (is this a relatively new process or an old one?) Explain 2. At the end of glycolysis where is most of the energy that was originally in glucose? How do we know? 3. Look at the glycolysis diagram again. Find Fructose 1,6-bisphosphate. How does the potential energy of this molecule compare to that of glucose? Explain. 4. Is glycolysis an aerobic process? (does it require oxygen?) 5. Knowing the ETC can convert the energy in 1 NADH into 3ATP, how many ATP will glycolysis ultimately yield if: a. You are an anaerobic cell? b. You are an aerobic cell? 6. Why is it significant that no special cellular structures are needed for glycolysis to occur? 7. How many carbons are present at the end of glycolysis? 8. We mentioned that cell respiration is sort of the “undoing” of photosynthesis. Specifically, what part of photosynthesis is glycolysis “undoing?” (THINK!!!) Pyruvate Oxidation 1. List what happens during the conversion of pyruvate into acetyl-coA. 2. How many of glucose’s carbons are left following pyruvate oxidation? 3. Pyruvate requires a transport protein to move it into the mitochondria. If a complex organism was born with a mutation such that it did not have this protein could it survive? Explain. Krebs Cycle 1. How many times must the Krebs cycle be completed to handle a glucose molecule? 2. Are the reactions that take place with the carbon compounds during the Krebs cycle exergonic or endergonic? Explain how we can tell? 3. Citric Acid is a 6 carbon compound. How many of these carbons came from glucose? 4. At the end of the Krebs cycle, where is the bulk of glucose’s energy? 5. In what ways is the Krebs cycle “undoing” the work of the Calvin cycle? List at least 4 specific things! The Electron Transport Chain of the Mitochondria 1. Draw a diagram of a mitochondrion and label the parts. (you may need to reference your text for this) 2. The cristae of the mitochondria are analogous to what structure found in plant cells? Explain. 3. Complete the following chart: ETC of the mitochondria What supplies the electrons for the reactions? Where do the electrons end up at the end? What does the movement of the electrons do? What does the movement of the H+ do? What reactant from the summary reaction is used here? What product from the summary reaction is made here? Light reactions of the chloroplast 4. What mechanisms are shared between the ETC and the light reactions? 5. Why would a lack of oxygen cause glycolysis and the krebs cycle to stop if oxygen isn’t used until the ETC? Anaerobic Respiration 1. Why is pyruvate such a significant molecule in the decision to proceed anaerobically vs. aerobically? 2. Most organisms (like yourself) that can do both anaerobic and aerobic respiration, prefer to do aerobic. Why? 3. All complex multicellular organisms are aerobic organisms. The only strictly anaerobic organisms are simple single celled organisms. Why does this make sense? 4. How many ATP can an ANAEROBIC organism get from a single molecule of glucose? 5. What process(es) produce(s) all of this ATP? 6. What is the purpose of fermentation? 7. What process in aerobic respiration accomplishes the same thing that fermentation does for the anaerobic organisms?