05extractionappendix..
advertisement

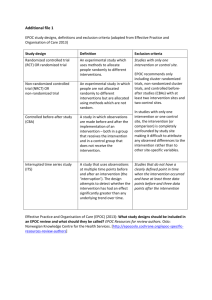
V.2.0 July 31 2009 A Systematic Review of Depression Management in Patients with Diabetes: Additional Data Extraction Information New to this version: - A new section, “Classifying interventions”, has been added. - Additional extraction instructions (section E) have been added. This guide is intended to supplement the instructions provided in the Data Extraction Form. It is divided into 4 sections: A. Classifying studies by design B. Classifying interventions C. The Cochrane Risk of Bias and EPOC Risk of Bias tools D. Using Downs and Black’s checklist E. Additional extraction instructions as agreed upon by reviewers Classifying studies by design - - Experimental studies and observational studies may be distinguished by question 1. Experimental studies may be classified as … o Randomized Controlled Trial (RCT) o Controlled Clinical Trial (CCT) o Controlled Before and After Study (CBA) … according to the definitions provided in the Cochrane EPOC Review Group “Data Collection Checklist” - Observational studies may be classified as prospective cohort study (PCS), retrospective cohort study (RCS), nested case-control study (NCC), or case-control study (CC). The guidelines provided in Chapter 13 of the Cochrane Handbook may be helpful. Guidelines for patient-allocated studies are reproduced below. A different table is available for cluster-allocated studies. Classifying interventions - - The schema used by Shojania et al. (2006) will be used here to classify interventions. Reviewers should select all the categories that apply, and, additionally, designate one particular category “primary”. At this time, the categorization of cognitive behavioral therapy (CBT) or similar components is unclear. Reviewers should note the presence of these components clearly to facilitate potential recoding. (July 31, 2009) A team change excludes the involvement of independent consultants by “referral”, which is an element of usual care. A team change must involve a substantive change to the organization of practice within a primary care group. Using Cochrane Risk of Bias and EPOC Risk of Bias tools - The EPOC modification to Cochrane’s Risk of Bias tool is included in the Quality Assessment form for general experience, and will not likely be included in the analysis. - Information on using Cochrane’s Risk of Bias tool is provided in Chapter 8 of the Cochrane Handbook. Useful tables and figures are reproduced below. - The EPOC modification is provided as follows. Using the Downs and Black Checklist - The primary quality assessment tool will be the Downs and Black Checklist. Instructions from the appendix of the 1998 validation study are provided below. - - - Question 5 requires a list of confounders. o Patient demographics: age, and gender. o Diabetes characteristics: duration of diabetes, number or nature of complications, physiologic control (i.e.: HbA1C) o Depression characteristics: Number of individuals with depression, baseline depression severity, presence of psychiatric comorbidities For an observational study, if the distribution at baseline of some but not all of the above confounders is provided, assign a score of “1” to question 5. For randomized controlled trials, assign a score of “2” if the study reports the baseline distribution of some potential confounders, and the distributions appear roughly equal between groups. The interpretation of question 27 is unclear. As a working provision, we will assign a score of “0” if no power calculation is provided, “3” if a power calculation is provided but the importance or impact of the difference between groups used in the calculation is unclear, and “5” if the difference between groups is clearly defined as a clinically important difference. This code rule may be subject to change, and reviewers may wish to quote from the report under comments to facilitate a future recode. Additional extraction instructions - - Because reviewers will have limited opportunities to come to agreement on important outcomes during the week of August 3-7, reviewers have agreed to extract all additional outcomes provided in study reports using a Supplemental Extraction Form. (July 31, 2009) Question 16 – Patient inclusion criteria: Reviewers should extract the essential criteria required to determine the disease and comorbidity statuses of patients, and whether patients belong to particular identifiable sub-groups (e.g.: linguistic, ethnic, or age-related groups). Criteria like “patients 18 and over with adequate hearing” should not be extracted. (July 31, 2009) Darren’s proposed additional extraction instructions (awaiting feedback) - Please provide F-statistics and T-statistics for outcomes, where specified. - Please note presence of “n” boxes in the outcomes tables, which may be used to calculate patient follow-up. If patients have crossed protocols and have been analyzed “as treated”, this should be noted in the final comments page of the extraction form.