2302169 Chem Med Studt แบบฝึกหัด Carbohydrate, Amino Acid and
advertisement

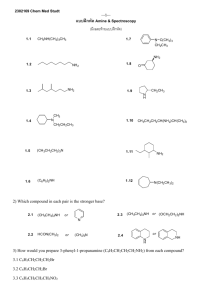
2302169 Chem Med Studt ---1--- แบบฝึ กหัด Carbohydrate, Amino Acid and Peptide (มีเฉลยบางข้ อท้ ายแบบฝึ กหัด) 1) Convert each compound to a Fisher projection and label each stereogenic center as R or S. Br COOH 1.1 H3 C Br H 1.2 H3 C H 3 CH 2C 1.3 H Cl 1.4 H 3CO OCH2 CH3 Cl 1.5 H Br CH3 H Br Cl H3 C CH2 CH3 H 1.6 H CH2 CH 3 Br Cl Cl H Br 2) For D-arabinose 2.1 Draw its enantiomer. 2.3 Draw a diastereomer that is not an epimer. 2.2 Draw an epimer at C3. carbonyl group. 2.4 Draw a constitutional isomer that still contains a 3) Draw a Haworth projection for each compound 3.1 β-D-talopyranose 3.3 α-D-galactopyranose 3.2 β-D-manopyranose 3.4 α-D-ribofuranose 4) D-Arabinose can exit in both pyranose and furanose forms. 4.1 Draw the α and β anomers of D-arabinofuranose. 4.2 Draw the α and β anomers of D-arabinopyranose. 5) Draw the products formed when α-D-gulose is treated with each reagent. OH 5.1 CH3I, Ag2O 5.4 C6H5CH2OH, HCl 5.2 CH3OH, HCl 5.5 Ac2O, pyridine OH O HO OH 5.3 C6H5CH2Cl, Ag2O D-gulose 6) What products are formed when each compound is subjected to a Kiliani-Fisher synthesis? CHO CHO 6.1 H H OH OH CH 2OH OH 6.2 HO HO H H H OH CH 2OH CHO 6.3 HO HO HO H H H H OH CH 2OH ---2--- 7) Identify the the compounds A-F in the following reactions. OH OH OH O 7.1 HO HO CH 3I O O HO HO OH A H3 O+ B + C + CH 3OH Ag 2O (Both anomers of B and C are f omed) OH OH O 7.2 CH 3I HO OH H3 O+ D E + F + CH 3OH Ag 2O O HO HO (Both anomers of E and F are fomed) HO O OH 8) Deduce the structure of the disaccharide isomaltose from the following data. [1] Hydrolysis yields D-glucose exclusively. [2] Isomaltose is cleaved with α-glucosidase enzyme [3] Isomaltose is a reducing sugar. [4] Methylation with excess CH3I, Ag2O and then hydrolysis with H3O+ forms two products: OCH3 H3CO H 3CO OH O CH3O OH H3CO H 3CO O CH3O OH 9) Draw the structures of each amino acid at its isoelectric point. 9.1 alanine 9.3 aspartic acid 9.2 methionine 9.4 lysine 10) Draw the major form of each of the following amino acid at pH = 1. 10.1 threonine 10.3 aspartic acid 10.2 methionine 10.4 arginine ---3--- 11) Draw the organic products formed in each reaction. O 11.1 NH3 (CH 3) 2CHCH 2CHCOOH excess Br O COOEt [3] H3 11.3 O OHC COOEt 11.5 COOEt [2] AcO CH2 Br O+, H H 3CO [1] NH 4Cl, NaCN [2] H3 O+ O [1] NaOEt 11.2 N H 11.4 N H COOEt [1] NaOEt [2] Cl(CH 2) 4NHAc [3] H3 O+, heat heat [1] NH 4Cl, NaCN NHAc [2] H3 O+ 12) Draw the structure of each peptide. 12.1 Phe-Ala 12.3 Lys-Gly 12.2 Gly-Gln 12.4 R-H 13) Name the peptide using both the three-letter and one-letter abbreviations of the component amino acids. H 2N H N O N H O H H CH 2CH 2COOH OH O COOH 14) Draw the s-trans and s-cis conformations of the peptide bond in the dipeptide Ala-Ala. 15) Draw all the products formed in the following reaction. O Boc N H OH + O H 2N DCC OH H CH(CH3 )2 16) Draw all the steps in the synthesis of each peptide from individual amino acids 16.1 Gly-Ala 16.2 Phe-Leu 17) Write out the steps for the synthesis of each peptide using the Merrifield method 17.1 Ala-Leu-Phe-Phe 17.2 Phe-Gly-Ala-Ile 18) Devise a synthesis of each amino acid from acetaldehyde (CH3CHO). 18.1 glycine 18.2 alanine ---4--- เฉลย Carbohydrate, Amino Acid and Peptide H Cl R Br H Cl S Br 1.6) 1.1), 1.3), 1.4) S; 1.2) R; 1.5) S,S; CHO H OH HO H 2.1) HO 2.2) H H CH 2OH CH 2OH O OH H 3.2) 2.4) O HO OH OH OH CH 2OH constitutional isomer diastereomer (but not epimer) CH 2OH O OH OH OH CH 2OH OH OH CHO H OH HO H 2.3) C3 epimer enantiomer 3.1) CHO HO OH HO H OH 3.3) OH OH CH 2OH O OH HOH 2C O 3.4) OH OH OH OH OH OH OH 4.1) HOH 2C OH OH O HOH 2C O OH OCH3 O 5.1) H3 CO H3 CO OH BnO O 5.2) + anomer HO HO + anomer 6.1) O 5.5) AcO 7.1) H 3CO OCH 3 O O H 3 CO H 3CO AcO OBn CHO H OH H OH + C2 epimer H OH CH 2OH H 3CO OCH 3 O 7.2) H 3CO H 3CO H 3CO OCH 3 O H 3CO OCH 3 H 3CO OCH 3 O O D H 3CO O CHO H OH HO H 6.3) HO H + C2 epimer HO H H OH CH 2OH OCH 3 O OH H 3CO HO H 3CO H 3CO H 3CO E OCH 3 HO H3 CO H 3 CO OH OCH 3 O H 3CO C B H 3CO H3 CO H 3 CO OAc CHO H OH HO H 6.2) HO H + C2 epimer H OH CH 2OH A H 3CO OBn OAc HO HO Bn = C 6 H5 CH 2- BnO BnO AcO 5.4) OBn O OCH 3 OH O OH OH anomer 5.3) HO OCH 3 OH OH OH anomer anomer anomer O OH OH OH OH OH OH H3 CO O 4.2) OH H 3CO O F OH OH ---5--H 3CO OCH 3 O 7.2) H 3CO H 3CO H 3CO O H 3CO O H3CO H 3CO HO OCH 3 O OH F OH 9.3) aspartic acid CO 2+H N C H 3 CH 2CH 2SCH3 CH 3 CO 2H 3N C H +H threonine 9.4) lysine CO 2H 2N C H CH 2CH 2CH2 CH 2 NH 3+ CO 2H N C H 3 10.3) pI = 5.41, +2 charge at pH 1 CO 2H H 3N C H aspartic acid CH 2CO2 H (CH 2) 3NH H2 N 11.1) (CH 3) 2CHCH 2CHCO2 NH 4 + NH 4 +Br - 11.2) HO NH 3 NH 2 O 11.3) HO2 C CO 2H 11.4) HO NH 2 NH 2 H 5C 6 H2 C O H N H 2N O 12.2) OH H N H 2N O CH 3 H N O H N 12.4) H2 N 14) CH3 CH3 13) Gly-Asp-Glu or G-D-E O OH N H H2 N O NH H 3C H3 C s-tr ans s-cis 15) Boc N H H N O OH O O O OH CH(CH3)2 + H 2N (CH3)2CH N H NH 2 CH 2CHCO2 H (CH 2) 4NH 2 H N C CO 2H 11.5) 2 H H 2N OH CH 2CH 2CONH2 N O NH 2 O OH H N O H2 N methionine CH 2CH 2SCH3 10.3) pI = 2.98, +1 charge at pH 1 NH 2 HO O 10.2) pI = 5.74, +1 charge at pH 1 CHOH CH 3 HN OH OH CH 2CO2 H 10.1) pI = 5.06, +1 charge at pH 1 12.1) O OH 9.2) methionine CO 2+ H3N C H CO 2+ H3N C H CO 2H H 3N C H HO HO OCH 3 9.1) alanine + 8) E D +H O H 3CO O H3CO H 3CO H 3CO H 3CO OH HO CH(CH3)2 OH O H N 12.3) H2N O O OH