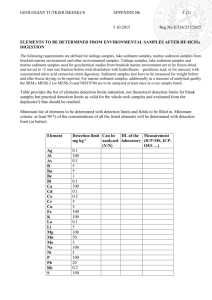
LAS
advertisement

REPORT The environmental fate of linear alkylbenzene sulphonates (LAS) and other anionic surfactants in sediments in the city of Stockholm and its inner archipelago. Using manganese speciation as a tool for assessing the role of surface sediment redox conditions for degradation of anionic surfactants. Organization IVL Swedish Environmental Research Institute Ltd. Report Summary Project title Address P.O. Box 21060 SE-100 31 Stockholm Project sponsor Telephone +46 (0)8-598 563 00 Author Title and subtitle of the report . Summary . Keyword Bibliographic data IVL Report The report can be ordered via Homepage: www.ivl.se, e-mail: publicationservice@ivl.se, fax+46 (0)8-598 563 90, or via IVL, P.O. Box 21060, SE-100 31 Stockholm Sweden . IVL report Contents . ......................................................................................................Error! Bookmark not defined. Introduction ........................................................................................................................................2 LAS ..................................................................................................................................................2 Other anionic surfactants .............................................................................................................3 Manganese as an indicator of past redox conditions in surface sediments ...........................4 Biogeochemistry of manganese in sediments ........................................................................4 Aim ..................................................................................................................................................8 Materials and methods ......................................................................................................................8 Sampling ..........................................................................................................................................8 Chemical analysis ............................................................................................................................ 10 Sediment dating ........................................................................................................................... 10 Sediment redox conditions ........................................................................................................ 10 Samples selected for the analysis of LAS and other anionic surfactants ............................ 11 Results............................................................................................................................................... 13 Sediment characteristics/dating ................................................................................................ 13 Sediment redox conditions ........................................................................................................ 13 Anionic surfactant concentrations ........................................................................................... 16 LAS ........................................................................................................................................... 16 Discussion ........................................................................................................................................ 23 Sediment characteristics and redox conditions ...................................................................... 23 Lake Mälaren ........................................................................................................................... 23 Saltsjön ..................................................................................................................................... 24 Torsbyfjärden .......................................................................................................................... 25 Trends between stations ........................................................................................................ 26 Spatial distribution of linear alkyl sulfonates .......................................................................... 27 Vertical Profiles and aerobic versus anaerobic degradation ................................................. 29 Ecotoxicity ....................................................................................................................................... 31 Conclusions...................................................................................................................................... 33 References ........................................................................................................................................ 34 Appendix .......................................................................................................................................... 39 1 . IVL report Introduction Surfactants are vital components of detergent formulations. Given the globally extensive use of washing detergents, it is not surprising that surfactants are among the most widely used chemicals in the world with a worldwide market of 24.33 billion US$ in 2009 (Acmite, 2010). The two dominating types of surfactants are nonionic and anionic, together making up of around two thirds of the global market value for surfactants (Acmite, 2010). The market value of nonionic surfactants has during the last 10 years become larger than that of anionic surfactants, and is now the largest of all surfactants whereas nonionic share of the world market was around 30% in 2009(Acmite, 2010). Global demand for anionic surfactants was 6.5 million tons in 2010 tons (Ceresana Research, 2012). The main use of surfactants is domestic use, around 70% as household cleaners and detergents and 9.5% as body care products and cosmetics in 2010 (Ceresana Research, 2012). Thus for these compounds wastewater is the main source to aquatic environment. Irrespective if it is in the marine, estuarine or lacustrine environment into which the surfactants are discharged, a relative rapid degradation in the water column follows. Because of the surfactants´ high affinity for organic carbon, the remaining load is eventually incorporated into the sediments. Accordingly one key property when assessing environmental risks of surfactants is biodegradation in water and sediment. Particularly, the ability of the surfactant to undergo biodegradation under all prevailing sediment redox conditions is of great interest. Since degradation in oxygenated sediment pore water is as rapid as in the water column, the focus has been on biodegradation under anoxic and anaerobic conditions. Anoxic conditions will prevail immediately below the oxic and suboxic layer, which exist in the top few millimeters if no bioturbation of the sediments occur and if transport of oxygen only occurs through diffusion from the bottom water. With the presence bioturbating meio and macro fauna, the pore water of the top centimeters of the sediments will be oxygenated. Eventually, with increasing sediment depth, strict anaerobic conditions exist. The question to what extent one of the main surfactants, linear alkylbenzene sulphonates (LAS) is biodegradable under anaerobic conditions, has attracted some attention from the scientific community, policymakers and environmental organizations, particularly in Sweden. Another key property is the toxicity of the surfactant itself and that of the metabolites produced during its degradation. However, several other aspects have to be regarded if one wants to assess the combined environmental impact of the whole life cycle of the surfactant. Some of these aspects include: washing efficiency, production of raw materials for the surfactant, production itself and properties important to the operations of wastewater treatment plants. Considering the vast volumes of surfactants used and their widespread usage, the selection of cleaning agent has to be selected carefully to ensure minimum impact on the total environment. LAS Linear alkylbenzene sulphonates (LAS) is globally the primary cleaning agent used in many washing detergents and cleaners. Detergents based on LAS were developed during the 1960’s to replace the branched tetrapropylenbenzensulfonates (TPBS), that due to high persitance caused considerable aggregations of foam in the water recipients. The LAS molecule contains an aromatic ring sulphonated at the para position and attached to a 2 . IVL report linear alkyl chain that typically has 10 to 14 carbon atoms. Concerning LAS, an extensive database of studies demonstrates rapid and complete (ultimate) biodegradation of LAS in many of the available aerobic biodegradation tests, including soil and the aqueous environment. In several tests, LAS has been shown to be readily biodegradable, and has passed the 10-day biodegradation window in mineralization tests for most ready tests. LAS is removed in biological wastewater treatment at percentages ranging from 77- 82% for trickling filters up to 99% for activated sludge (OECD 2005). During the 80’s 10000-15000 tonnes of LAS were used per year in detergents in Sweden (KEMI, 2012a). However, ecolabeling organizations, such as Good Environmental Choise (Bra Miljöval), Nordic Ecolabel (Svanen) and EU-Ecolabel have not apporved LAS in laundry detergents based on a too low anaerob degradation rate. The critera set are, for instance, that ”60% of the cleaning agent must be anaerobially degraded according to ISO 11734 or another equivalent test”. (Naturskyddsföreningen, 2006) or ”All surfactants must be anarobically and aerobially biodegradable” (Nordic Ecolabelling, 2011). Sweden is the only country where ecolabeling of detergnts has considered the anaerobic degradation as the determining criteria for acceptance. As a consequence, in Sweden the use of LAS decreased with 95% between the years 1989 and 1999, compared to a decrease of 15% in other European countries. The Swedish Society for Nature Conservation estimated a decrease in usage of LAS in household chemicals from 6300 tonnes for the year 1988 to 260 tonnes in 1996 (SSNC, 1999). The import of LAS in all chemical products has increased during recent years, from 407 tonnes in 2001 to 1048 tonnes in 2007 (KEMI, 2012a). LAS is also imported as raw material (248-564 tonnes per year) and produced in Sweden. Information on the total amount of raw material produced and used in Sweden is however not publicly available. In other European contries as well as USA, South America and Asia, LAS is the primary cleaning agent due to its advantages compared to other anionic surfactants. LAS enables the possility to lower the washing temperature from 60 ºC to 30 ºC for most type of materials, that consequently saves energy for the households. With the use of LAS a smaller dose of washing detergent per volume of laundry can be used. A lower consumption of detergent will in turn reduce the transports which is a gain for the climate. However, LAS is manifactured through sulphonication of linear alkylbenzene (LAB). LAB is derived from benzene and linear paraffins both of which are petroleum derivatives. Thus LAS is not from a renewable source but from a petrochemical feedstock. Other anionic surfactants For the industry there is no rationallity in developing specific detergents that does not contain linear alkylbenznene sulphonates LAS for the Swedish, and possibly the Finish, market. Furthermore, the impact on third coutries needs to be considered. The anionic surfactants used in detergents on the Swedish market are primarly the alkyl ether sulphates (AES) which are sodiums salts of alkyl polyethylene ether sulphates e.g. sodium lauryl ether sulphate(SLES) and alkyl ether sulphates, e.g. sodium dodecyl sulphates (SDS)/sodium lauryl sulphates (SLS)and soft soap. These surfactants can also be derived from different 3 . IVL report types of oleochemical feedstock, generally palm oil and palm oil derivatives, pine needle oil or from animal residues and can, thus, come from renewable sources. However, the production of palm oil and palm oil derivatives have resulted in large global environmental consequences. To give space for palm oil plantages large areas of tropical rain forests in Indonesia (Borneo), Philipines and Malaysia have been devastated. The destruction of the rain forests have adverse impact on both the biodiveristy of species and the climate. At present only a few percent of the production of palm oil producion in the world is certified according to Roundtable on Sustainable Palm Oil (RSPO). In addition, an increased demand for palm oil will compete with the local food production and may make Indonesia, Malaysia and the Philipines more dependet on imported food. The properties for the anionic surfactants used are not as known as for LAS, e.g. concerning toxicity and bioaccumulation (Woldegiorgis, 2011). These surfactants, e.g. AES, are easily degraded in anaerobic standard tests but still detected in both freshwater and marine sediments (LaraMartin et al., 2006). For sodium dodecyl sulphate and sodium dodecyl ether sulphate usage in household products was estimated to 1600 tonnes in 1988 and 5600 tonnes in 1996 (SSNC, 1999). The import of sodium dodecyl sulphate in chemical products was 2191 tonnes in 2007 (KEMI, 2012b), of sodium dodecyl ether sulphate, 430 tonnes in 2009 (KEMI, 2012c), and of coco amidopropyl betaine, 289 tonnes in 2005 (KEMI, 2012d). Manganese as an indicator of past and present redox conditions in surface sediments By studying the changes in speciation (different chemical forms) of Mn with sediment depth, information on past bottom water and surface sediment redox conditions will be gained (Calvert et al., 2001 Morford et al., 2001). In order to understand how Mn speciation can be used, a summary of the biogeochemistry of manganese in sediments follows. Biogeochemistry of manganese in sediments A schematic figure of Mn-cycle in marine sediments is shown in Fig 1 below. 4 . IVL report Fig. 1. Schematic figure of Mn –fluxes in marine sediments (Wang and Van Cappellen, 1996). The sizes of the arrows represent the magnitude of the fluxes. The relative sizes of these fluxes depend on a number of factors, e.g. whether there is bioturbation or not and salinity (marine, brackish, lacustrine), and can therefore vary from location to location. Dissolved Mn is scavenged out of the oxygenated bottom water and deposited to the sediment surface by the adsorption to FeOOH and other particles (Turner and Millward, 2000) and by the adsorption to and precipitation of oxyhydroxides, MnOOH) and oxides MnO2 (Reed et al., 2011). Manganese oxyhydroxides and oxides are however mainly formed in the oxic upper part of the sediment from upward diffusing dissolved manganese, Mn2+ (Berner, 1980; Mouret et al., 2009). The dissolved manganese is also adsorbed to the manganese oxyhydroxides and oxides (Canfield et al., 1993). The dissolved manganese originates where Mn (III,IV) is reduced to Mn(II). The reduction of manganese occurs as part of a sequence of well-established diagenetic reactions. This sequence has a vertical distribution determined by preferential use of electron that yields the highest amount of free energy for the bacterially mediated oxidation of organic matter. At the sediment-water interface, oxygen is reduced, followed by the reduction of nitrate and manganese oxide, 5 . IVL report then reactive iron oxyhydroxides, FeOOH and finally the reduction of sulphate (Berner, 1980; Tromp et al., 1995; Hyacinthe et al., 2001). In addition to the redox reactions involving the microbial degradation of organic matter there as some important secondary redox reactions. Nitrate can oxidise upward diffusing Mn2+ and MnO2 is formed (Aller et al., 1998). Mn-oxides and Mn-oxyhydroxides can be reduced by upward diffusing Fe2+(Hyacinthe et al., 2001). They can also be reduced by ammonia forming either nitrate under anaerobic conditions or dinitrogen under aerobic conditions (Aller et al., 1998). Depth sequence of bacterially-mediated oxidation of organic matter (Hyacinthe et al., 2001) O.M. = C106H263O110N16P 1. Oxygen consumption by oxic respiration and nitrate production 138O2+ O.M. + 18HCO3-→124CO2 + 16NO3- + HPO42- + 140H2O 2. Nitrate consumption by denitrification 94.4 NO3- + O.M.→13.6 CO2 +92.4 HCO3- + 55.2N2 + 84.8H2O + HPO42 3. Reduction of Mn oxides by anaerobic respiration 236MnO2+O.M.+364CO2+104H2O→470HCO3-+8N2+236Mn2++HPO424. Reduction of Fe-oxides and production of ammonia 424Fe(OH)3+O.M.+740CO2→846HCO3-+424Fe2++16NH3+320H2O+HPO425. Production of sulphide and ammonia by sulfatoreduction 53SO42-+O.M.→39CO2+67HCO3-+16NH4++53HS-+39H2O+HPO42Secondary redox reactions coupled to iron and nitrogen (Hyacinthe et al., 2001): 6. Production of nitrate by nitrification: NH4++2O2→NO3-+2H++H2O 7. Oxidation of Mn2+ with oxygen 2Mn2++O2+2H2O→2MnO2+4H+ 4Mn2++O2+6H2O→4MnOOH+8H+ 8. Oxidation of Mn2+ with nitrate 5Mn2++2NO3-+4H2O→5MnO2+N2 +8H+ 9. Oxidation of Fe2+ with nitrate 5Fe2++NO3-+12H2O→5Fe(OH)3 +1/2N2+9H+ 10. Oxidation of Fe2+ with Mn-oxides Fe2++MnOOH + H2O→Fe(OH)3 + Mn2+ 2Fe2++MnO2+4H2O→2Fe(OH)3 + Mn2+ + 2H+ 11. Reduction of Mn-oxide by ammonia to give dinitrogen 2MnOOH+NH4++3H+→2Mn2++1/2N2+4H2O 3/2MnO2+NH4++2H+→3/2Mn2++1/2N2 + 3H2O 12. Reduction of Mn-oxide by ammonia, production of nitrate 8MnOOH+NH4++14H+→8Mn2+ + NO3-+13H2O 4MnO2+NH4+ + 6H+→ 4Mn2++NO3- + 5H2O Secondary redox reactions of carbon and sulphur (Wang and Van Cappellen, 1995) 6 . IVL report 13. 14. 15. 16. 17. 18. 19. 20. ≡S-Mn+ + ½ O2 + OH- → ≡S-H0 + MnO2 ≡S-Fe+ + ¼O2 + 3/2H2O + OH-→ ≡S-H0 + Fe(OH)3 H2S + MnO2 → Mn2+ + S0 H2S + 2O2 → SO42- + 2H+ H2S + 2Fe(OH)3 → 2Fe2+ + So + 4OH- + 2H2O FeS + 2O2 → Fe2+ + SO42CH4 + 2O2 → CO2 + 2H2O CH4 + CO2 + SO42- → H2S + 2HCO3- There are also adsorption reactions, which influences the concentrations of the dissolved species of nitrogen, iron and manganese (Wang and Van Cappellen, 1996): 21. NH4+↔ NH4+ (ads) 22. ≡S-H0 + Mn2+ + OH- ↔ ≡S-Mn+ + H2O 23. ≡S-H0 + Fe2+ + OH- ↔ ≡S-Fe+ + H2O Below the zone of sulfatoreduction in marine sediments, where sulphate is exhausted, the decomposition of organic matter continues with the production of methane (Berner, 1980, Tromp et al., 1995). This diagenetic reaction yields the least amount of free energy (Tromp et al., 1995) and occurs only if sulphate is not present. In the case of lake sediments and brackish water where the supply of sulphate is less, methane can be produced just below the sediment (Berner, 1980; Whiticar, 1999; Thiessen et al., 2006). 24. Production of methane (Wang and Van Cappellen, 1996) O.M. + 14H2O → 53CH4 + 35CO2 + 14HCO3- + 16NH4+ + HPO42In the anoxic part of the sediment the reduced manganese can precipitate as authigenic minerals, predominantly rhodocrosite, MnCO3 (Berner, 1980; Wang and Van Cappellen, 1996). This reaction and that of the iron analogue (siderite) and iron sulphide are three important precipitation and dissolution reactions influencing the concentrations of dissolved iron and manganese. Precipitation and dissolution reactions (Wang and Van Cappellen, 1996) 25. Mn2+ + 2HCO3- ↔ MnCO3 + CO2 + H2O 26. Fe2+ + 2HCO3- ↔ FeCO3 + CO2 + H2O 27. Fe2+ + 2HCO3- + H2S ↔ FeS + 2CO2 + 2H2O 7 . IVL report The precipitation of a mixed Ca-Mn solid carbonate seem to control the concentration of dissolved Mn2+ in the pore water (Magen et al., 2011). However, in coastal sediment with high concentrations of organic matter and low carbonate contents, complexation with dissolved organic matter can lead to porewater concentrations well above supersaturation with respect to MnCO3 (Mucci and Edelborn, 1992). The dissolved organic matter (DOM), produced during the microbial degradation of sediment organic matter, interferes with the nucleation and growth of MnCO3 (Berner, 1980). The rate at which manganese is reduced, and hence the preservation of Mn(IV,III) solid phases such as pyrolusite and manganite , in anoxic environments seems to be a complex function where the supply of fresh organic matter can be limiting (Fischer et al., 2008; Magen et al., 2011). Also, in low sulphate environments such as in lakes and brackish environments, mixed Mn(II/III)-Fe(II/III)-P solid phases formed in the oxic part of these sediment can remain stable as they become buried in the deeper, anoxic part of the sediments (Hyacinthe and Van Cappellen, 2004). If the concentrations of sulphates are higher, as in the marine environment, the ferric iron form iron sulphides instead. Aim The aim of this project is to get better knowledge concerning the occurrence and environmental fate of linear alkylbenzne sulphonates (LAS) for risk assessments in aquatic envrionmnents. The specific need for an improved basis for sediment risk assessment in Sweden has been identified by Woldegiorigis (2011). The result may contribute to a better contribution for regulation and ecolabelling of washing detergents and other products where surfactants are important components. Specifically, to assess the role of surface sediment redox conditions on the degradation of LAS is a major objective. Materials and methods Sampling The locations for sediment sampling were chosen in order to reflect the influence of two factors. Firstly, the impact from the city of Stockholm on the total loads of linear alkyl sulphonates (LAS) and other anionic surfactants and, secondly, the influence of sediment redox conditions on the degradation of LAS at the time of deposition. The total load of LAS and other anionic surfactants were estimated by taking sediment samples along a transect: Lake Mälaren, upstream of assumed major sources, in Saltsjön immediately downstream of city of Stockholm’s two WWTPs, (Bromma and Henriksdal) and finally in Torsbyfjärden, approximately 20 km further downstream. The influence of sediment redox conditions on the degradation of LAS was estimated by choosing two adjacent sampling 8 . IVL report points at each location with oxic and anoxic/suboxic condition, respectively, at the sediment surface. Below the mixing (bioturbation) depth all sediments are anoxic. The coordinates for the sampling locations are shown in Table 1 below Table 1. Locations, water depths and redox conditions at the surface of the sediment samples. Area Lake Mälaren M1 M2 Saltsjön S1 S2 Torsbyfjärden T1 T2 Oxic Anoxic Anoxic Oxic Anoxic Oxic Depth Coordinates 19 25 32 20 49 28 59 19.24; 18 00.03 59 19.18; 18 00.703 59 19.01; 18 06.78 59 19.23; 18 08.45 59 21.65; 18 26.86 59 20.53; 18 27.40 Location in relation to the WWTPs upstream upstream At WWTPs At WWTPs 20km downstream 20km downstream Fig. 2.Map over Stockholm area with Lake Mälare to the West and Stockholm Archipelag in the Baltic Sea to the East. Sediment sampling locations are marked with red dots. At each location two, in total 12, sediment cores were collected using a Kajak type gravity corer of plexi glass; length 50 cm and diameter 80 mm. Upon retrieval both ends of the sampling cylinder were sealed with air tight rubber caps and transported to the laboratory the same day as the sampling took place. In laboratory the length of sediment cores were measured and described. Two different clean up procedures followed depending on the coming analyses, either speciation of Mn to assess sediment redox conditions, or counting of 137Cs-activity for sediment dating. 9 . IVL report Chemical analysis Sediment dating One sediment core from each sampling point was analyzed for 137Cs-activity for sediment dating, estimated based on the peak in deposition of 137Cs in 1986 after the Chernobyl accident. The overlying bottom water was siphoned off and the top 10 cm of the sediment core was sliced in 2 cm sections. The rest of the sediment core was sliced in 1cm sections. The wet sediment samples were weighed and frozen. The frozen sediment samples were freeze-dried and weighed. The water content and porosity of the sediment core were calculated from the difference in the wet and dry weights. Each sample was grinded manually with a mortar and pestle. The organic material and the grain size distribution of the sample were classified by ocular inspection and containers were filled with clay, silt and sand material. The activities of the samples were counted in a gamma counter, Intertechnique CG 4000 with a 3´´ NaI detector. The measurement time was 100 min. Sediment redox conditions Two different methods were employed to discriminate between oxic and anoxic conditions in the upper 0.1m of the sediment core. The first method to discriminate between the different redox conditions of the surface sediments is ocular inspection of the core upon retrieval. Through the transparent plastic cylinder of the sediment core, the colour of the sediment and the presence of bioturbating animals and their burrows can been seen. In the case of an anoxic environment the sediment has an overall black colour with lighter and darker bands (lamina) representing winter and summer periods, respectively. The black colour comes from the presence of reduced organic material and sulphides. There can also be a smell of hydrogen sulphide (H2S), a gas formed during the decomposition of organic matter, which contains sulphur, under reducing conditions. Sometimes one can see a metallic lustre, coming from sulphur (formal oxidation state -2) in the form of sulphides. The sulphides constitute a solid and very stable mineral phase formed by sulphur under reducing conditions with a doubly charged metal cation such as Fe2+, Zn2+ and Cu2+. The winter bands are lighter in colour due to the higher proportions of clay minerals to reduced organic matter and sulphides. In contrast, a sediment sample from an oxic environment is grey to olive green – brown, depending on the relative amount of inorganic material, clay and hydrous iron oxide minerals, and organic material. Normally these sediment samples are not laminated because of the presence of bioturbating macro fauna. 10 . IVL report Below the level of 0.1m in the sediments in the area of study, the sediments are always anoxic because of the lack of oxygen diffusion even with the presence of bioturbating macro fauna. Macro fauna such as priapulid worm Halicryptus spinulosus, Baltic clam Macoma baltica and the amphipod crustacean Monoporeia affinis have their bioturbation depths confined to the upper 10cm of the sediments in hypoxic sediments (Bradshaw et al., 2006). The recent past and present redox conditions of the sediment samples were also analysed by studying the speciation of manganese (Mn). Mn can occur in different solid phases in the sediments, mainly as carbonates, oxides and oxyhydrooxides. The distribution of the two groups of Mn-species (II and III/IV) is operationally defined as the amount of Mn being extracted by 1 N HCl, Mn-HCl (both groups) and by ascorbate, Mn-Asc (only the most easily reduced group, III/IV). From each station one sediment core was analysed for speciation of Mn. The bottom seal of the sediment core was removed and the top seal was briefly opened to directly transfer and distribute the bottom end of the sediment core to shorter sampling pipes with the same diameter, but varying lengths; 2, 3, 5 and 8 cm, respectively. The subsamples had air tight rubber seals in the bottom and, after filled with sediment, capped with plastic film. The subsamples were directly transferred to an anaerobic (90% N2, 5% H2 and 5% CO2) chamber. In the anaerobic chamber, an equal volume of representative sediment samples for pore water analysis were taken from each subsample, transferred to 15 ml centrifugation tubes, capped and immediately centrifuged at 1500 rpm for 10 minutes. The pore water samples were transferred to glass tubes after the centrifugation, acidified with HCl to pH 2, and sealed with air-tight caps. The remaining sediment samples were put in pre-weighed plastic containers, weighed, frozen and thereafter freeze-dried. The freezedried sediment samples were weighed and the samples’ porosities and water contents were calculated as the difference between wet and dry weights. Two subsamples of 100 mg each was retrieved from every freeze-dry sediment sample and put in a glass tube with air-tight seals. One of the subsamples was extracted with 10 ml 1 N HCl and the other with 10 ml ascorbate. One litre of ascorbate solutions was prepared by dissolving 50 g NAHCO3, 50 g Na-citrate and 20 g ascorbic acid, buffered at pH 8. Both fractions (Mn-HCl and Mn-Asc) were shaken continuously for 24 h at room temperature. For the HCl extraction the supernatant was thereafter diluted with water and the ascorbate extraction with 0.2 M HCl. Mn in pore water and extraction samples was measured with flame atomic absorption spectroscopy, using an external aqueous standard for calibration. Mn-HCl represents the whole fraction of Mn-oxides and Mn associated with carbonates. Mn extracted with ascorbate is the most reducible part of Mn (III, IV) oxides and oxyhydroxides. Samples selected for the analysis of LAS and other anionic surfactants 11 . IVL report Based on the results from the sediment dating, sediment sections representing recent years (surface sediment), the mid 1980s (peak in Cs137) and sections between these periods, were chosen for anionic surfactant analyses. At the sampling stations where the sedimentation rates were high (S1) or low (T1 and T2), sections deposited before the peak in Cs137 were also sampled. In the former case the high sedimentation rate allowed for expanding the time period studied, while keeping the same number of analyses. In the latter case, the sectioning of the sediment cores were not fine enough to resolve the period in between sedimentation during recent years and the peak in Cs137. In the sediment cores sampled at M1 and S2, bioturbation had occurred constantly and therefore impossible or difficult to identify a peak in Cs137activity. Thus, for these sediment cores, sections were chosen based on sedimentation rates inferred from nearby sites in this study and others (Östlund et al., 1998 and Jönsson, 2011). Due to that larger sections were used for the analysis of sediment redox conditions, the results from sediment dating from the replicate cores could not always be matched. The first analysis of the sediment cores revealed concentrations of individual linear alkyl sulphonates (LAS) homologs above µg/g dw for the sediment cores sampled in Saltsjön, which is above the concentration linear range of the analytical instrument (UFLC-MS/MS) used to quantify the individual anionic surfactants. Therefore a second analysis focusing on the content of LAS in the Saltsjön sediment cores were performed, in which either sample extracts from the first set of analysis of Saltsjön were diluted or samples representing new depths in the Saltsjön sediment cores were prepared with a lower level of preconcentration. Also, samples representing resembling or new depths in the Torsbyfjärden sediment cores were analysed to verify differences in content of LAS between sediment cores sampled at the same location. Sample extraction and clean-up Three gram of freeze-dried sediment was spiked with 4-octylbenzenesulfonic acid (C8LAS) as surrogate standard and extracted twice with methanol: water (1:1). The supernatants were combined and loaded on a SPE-cartridge containing graphitized carbon as adsorptive phase. Prior to elution, the SPE-cartridge was washed with methanol. The analytes were eluted from the cartridge utilizing 10 mM tetramethylammonium hydroxide in dichloromethane: methanol (1:1). The elute was evaporated to dryness under a gentle stream of nitrogen at 40 °C and reconstituted in 0.5 ml methanol: water (1:1). Instrumentals A binary liquid chromatography (UFLC) system with auto injection (Shimadzu, Japan) was utilized for injection of samples and pumping of the mobile phase. The chromatographic separation was performed on a C8- reversed phased column (dimensions 50 x 3 mm, 5 µm particle size) (thermo scientific) at a temperature of 35°C. The mobile phase consisted of 10 mM ammonium acetate in water (A) and methanol (B). The system was programmed to deliver a linear gradient with an initial composition of 30% B, which was kept for 2 min. After 2 min the composition of B was increased from 30% to 100% in 6 min. This composition was kept for 3 minutes and thereafter returned to 30% B. The total run-time 12 . IVL report was 15 min and the flow rate was 0.4 ml/min. The UFLC-system was coupled to an API 4000 tipple quadrupole (MS/MS) (Applied Biosystems) with an electrospray ionization interface (ESI) operated in negative ion mode. Results Sediment characteristics/dating The Cs137-activity data in the sediment cores from Mälaren, M1 oxic, (see appendix) had a weak increase down to a depth of six cm followed by a weak decrease down to eight cm. The diluted Cs137-activity depth profile indicates that the sediment has been bioturbated. Thus the deposition rate cannot be determined. At the other site, M2 anoxic, the Cs137activity data indicate no or possibly very little bioturbation. A rather well defined peak in Cs137-activity at 15 cm depth corresponds to an estimated annual average dry matter deposition rate of 0.26 g/cm2. In Saltsjön, the average annual dry matter deposition rate at S1 anoxic was estimated to be 0.21 g/cm2, assuming that the 15 cm sediment depth correspond to the year 1986. For the other sediment core, S2 oxic, a maximum in Cs137-activity cannot be defined due to a continuous bioturbation. The annual average dry matter deposition rates in Torsbyfjärden at T1 anoxic was estimated to be 0.18 g/cm2. Since the year 1986 ten cm of sediment was estimate to have been deposited, as indicated by the Cs137-activity data. The corresponding depth at the other site, T2 oxic, was six cm, which gives an estimated annual dry matter deposition of 0.11 g/cm2. Sediment redox conditions The manganse data from Mälaren, M1 oxic, (Fig. 3) indicated a redox-boundary between 5 and 10 cm. Above 10 cm sediment depth there was an increase in Mn-Asc up to the sediment –water interface. Below 10 cm sediment depth the Mn-Asc inventory remained the same whereas Mn-HCl increased somewhat. The porewater Mn2+ concentrations at M2 anoxic (Fig. 3) did not vary with sediment depth and was approximately 20 moles. The Mn-HCl concentrations vary only to a relative small extent and no clear trend is discernible. At M2 the distinct positive increase in concentrations of Mn-Asc close to the sediment surface could indicat a redox-boundary somewhere in this interval (0-8 cm). However this is probably not the case due to the negative difference between the two fractions Mn-HCl and Mn-Asc and the decoupling of their variations with sediment depth. 13 . IVL report The sediment profiles of Mn-Asc and Mn-HCl in Saltsjön, S1 anoxic (Fig. 4) displayed a distinct positive difference between the Mn-HCl and Mn-Asc concentrations. However, below 10 cm sediment depth, this had changed to a negative difference as in the samples from M2 in Lake Mälaren. The Mn-Asc data from S2 oxic (Fig. 4) displayed a peak in the Mn-Asc inventory at a sediment depth of 3-4 cm and then stabilised at approximately 10 moles/g. The corresponding Mn-HCl inventory showed an increase from the sediment surface down to 10 cm. The Mn-HCl inventory then remains steady at approximately 19 moles/g. Once again, there is a difference of Mn-HCl and Mn-Asc in the top 4 cm. The sediment depth profile of porewater Mn2+ concentrations showed a peak at 3-4 cm, followed first by a decrease and then an increase. The Mn-data from station T1 anoxic in Torsbyfjärden (Fig. 5) displayed a maximum in the Mn-Asc inventory in the top 4 cm. Below the Mn-Asc inventory decreased down to 14 cm and thereafter increased down to 19 cm, where it stabilised at approximately 30 moles/g. There was a simultaneous increase in the porewater Mn2+ concentration with depth below 10 cm. At T2 oxic, the sediment Mn-data (Fig. 5) indicated a weak increase in both Mn-Asc and Mn-HCl with sediment depth. The inventories of Mn-Asc and Mn-HCl in mole/g are smaller compared to T1. Fig. 3. Sediment profiles of Mn-Asc, Mn-HCl (mole/g) and Mn2+ (mole) in Lake Mälaren. 14 . IVL report 0 Mn-Asc, Mn-HCl (uM/g); Mn2+ (uM) 10 20 30 40 50 0 Sediment depth (cm) 5 S2: Mn-Asc S2: Mn-HCl 10 S1: Mn-Asc 15 S1: Mn-HCl S2: Mn2+ 20 25 30 Fig.4. Concentrations of Mn-Asc, Mn-HCl (mole/g) and Mn2+ (mole) in sediment samples from Saltsjön. 0 Mn-Asc, Mn-HCl (uM/g); Mn2+ (uM) 20 40 60 80 0 Sediment depth (cm) 5 T2: Mn-Asc 10 T2: Mn-HCl T1: Mn-Asc 15 T1: Mn-HCl 20 T1: Mn2+ 25 30 35 Fig. 5. Sediment profiles of Mn-Asc, Mn-HCl (mole/g) and Mn2+ (mole) in Torsbyfjärden. 15 . IVL report Anionic surfactant concentrations LAS Linear alkylbenzene sulphonates (LAS) was found in samples from all sampling sites and at all sediment depths analysed (Figs. 6-11). In both anoxic and oxic sediment cores from the eastern part of Lake Mälaren, the concentrations of LAS were in the range 82-440 ng/g dw, (Fig. 6 and Fig. 7). Slightly higher concentrations were found in the deepest sediment sections but no clear vertical distribution pattern could be seen. 0 Concentration (ng/g dw) 200 400 0 Concentration (ng/g dw) 100 200 300 400 Sediment depth (cm) C10-LAS 0_7 0_4 C11-LAS C12-LAS 8_12 7_12 12_17 12_17 C13-LAS Fig.6. Las concentrations in sediments at M2 (anoxic) Sediment depth (cm) 0 Concentration (ng/g dw) 50 100 150 200 0 Concentration (ng/g dw) 200 400 600 0_2 2_4 0_6 4_6 Fig. 7. LAS concentrations in sediments at M1 (oxic) 16 C10-LAS C11-LAS C12-LAS C13-LAS . IVL report In sediment cores sampled in Saltsjön, LAS concentrations were higher than in Mälaren, with the highest concentrations in the anoxic cores, (Fig. 8 and 9). In surface sections from the oxic cores (S2), concentrations of 3900 and 7900 ng/g dm were found, whereas surface sections from the anoxic cores (S1) contained 7000 and 12000 ng/g dm. Highest concentrations were found in the deeper sediment sections with concentrations of 8300 and 11000 ng/g dm in the oxic cores and even higher concentrations in the anoxic cores, 32000 and 44000 ng/g dm. As could be expected, the difference in concentrations between surface sediments and deeper sections were more profound in the anoxic cores. In the oxic cores, concentrations in deeper sediments were 1.4 and 2.1 times higher compared to surface sediment. In the anoxic cores, the deeper sediments contained 2.6 and 6.2 times higher concentrations. Sediment depth (cm) 0 Concentration (ng/g dw) 20000 40000 0 0_2 0_5 6_8 5_10 12_15 10_15 Concentration (ng/g dw) 20000 40000 60000 15_18 C10-LAS C11-LAS 18_21 C12-LAS C13-LAS Fig. 8. LAS concentrations in sediments at S1 (anoxic). Sediment depth (cm) 0 Concentration (ng/g dw) 5000 10000 15000 0 0_2 0_2 2_6 2_6 6_10 6_11 10_12 14_16 Fig.9. LAS concentrations in sediments at S2 (oxic). 17 Concentration (ng/g dw) 5000 10000 C10-LAS C11-LAS C12-LAS C13-LAS . IVL report Sediment depth (cm) 0 Concentration (ng/g dw) 10 20 30 40 0 0_2 Concentration (ng/g dw) 100 200 300 0_4 2_14 4_14 C10-LAS C11-LAS C12-LAS C13-LAS Fig. 10. LAS concentrations in sediments at T1 (anoxic). Sediment depth (cm) 0 Concentration (ng/g dw) 500 1000 0 1500 0_2 0_5 2_6 5_10 Concentration (ng/g dw) 50 C10-LAS C12-LAS 100 C11-LAS C13-LAS Fig. 11. LAS concentrations in sediments at T2 (oxic). In the sediment cores from Torsbyfjärden, the concentrations of LAS were significantly lower on a sum-basis as well as for the homologs C12 and C13 (Fig. 10 and 11) compared to Saltsjön (Fig. 8 and 9). For surface sediments from both the oxic and anoxic site, the concentrations of C10 and C11 homologs were about the same magnitude as in Saltsjön. Overall, the distribution of LAS concentrations with sediment depth and between oxic and anoxic sites displays a relative large heterogeneity or variance. To test if there were any significant differences in mean concentrations in surface sediments of linear alkylbenzene sulphonates (LAS) depending on redox environment (oxic/anoxic) or distance from WWTPs (Saltsjön/Torsbyfjärden) a series of pairwise t-tests were performed using both sum- and C10-C14-LAS concentration data. The results from these tests are shown in tables 2 and 3 below. 18 . IVL report Table 2. Pairwise t-test to test differences between mean concentrations of sum, C10, C11, C12 and C13 LAS in oxic and anoxic surface (0 – 7 cm) sediments. Anox/Ox C10 C11 C12 C13 Sum C10 0.56 C11 C12 C13 Sum 0.52 0.83 0.91 0.83 Table 3. Pairwise t-test to test differences between mean concentrations of sum, C10, C11, C12 and C13 LAS in Saltsjön (anoxic+oxic) and Torsbyfjärden (anoxic + oxic) surface (0 – 7 cm) sediments. Salt/Tor C10 C11 C12 C13 Sum C10 0.15 C11 C12 C13 Sum 0.02 3e-4*** 0.00085*** 0.00042*** *** 99.9 % significance 19 . IVL report Alkyl sulphate, alkyl ether sulphates and cocoamidopropyl betaine The concentrations of the anionic surfactants alkyl sulphate (AS) and alkyl ether sulphate(AES) were below their respective detection limit (LOD) in the majority of the sediment samples investigated (Appendix A6-A7). The concentrations of cocoamidopropyl betaine (CAPB) were below the LOD in all of the sediment samples (Appendix A6). The concentrations of the anionic surfactants AS, AES and CAPB are presented in the figures below (Figs 12 – 17). Values below LOD are represented by half their respective LOD. Detectable concentrations of AS were found in surface sediments at all sites except T2, which was an oxic site. The concentrations were clearly highest at S1 and T1, both anoxic, although the variance between cores and samples of the same core with depth was once again clearly higher at T1. Only at S1 could AS be detected at sediment depths greater than 10cm or approximately the zone of bioturbation. All four ethersulphates (1,2,3,4-AES) could only be detected in the top 10cm at S1 (anoxic) while 1,3,4-AES could be detected in the sample from 10 – 15cm of sediment depth at S1. Thus the parameter sum-AES could was only above its’ LOD in the top 15cm at S1.There was however an inter-core variance at S1, since in the other core, only 1,3-AES could be detected in the surface sample (0-2cm) and 1,4-AES in a deeper sample (6-8cm). In the sample from 12-15cm only 4-AES could be detected in this core. At the other anoxic sites, 3,4-AES could be detected in samples T1 (0-4; 4-9cm) and M2 (0-7cm). At oxic sites only 4-AES could be detected at M1 (0-2cm) and T2 (2-4cm). Fig. 12. The bars represent concentrations of Alkyl sulphates (AS), alkyl ether sulphate (AES) and cocoamidopropyl betaine (CAPB) in sediments at M1 (oxic). The concentrations represent half their respective limit of detection (LOD) with the exception of AS in the 0 – 2cm sample from core 1 used for dating by Cs137 (left panel). 20 . IVL report Fig. 13. The bars represent concentrations of alkyl sulphates (AS), alkyl ether sulphate(AES) and cocoamidopropyl betaine (CAPB) in sediments at M2 (Anoxic). The concentrations represent half their respective limit of detection (LOD) with the exception of AS in the 8 – 12cm sample from core 2 used for dating by Cs137 (left panel). Fig. 14. The bars represent concentrations of Alkyl sulphate (AS), alkyl ether sulphate(AES) and cocoamidopropyl betaine (CAPB) in sediments at S1 (Anoxic). All concentrations of CAPB, of AS in sample 12-15cm from core 2 and all of AES from core 2 used for dating by Cs 137(left panel), are represented by half their respective limits of detection (LOD) Fig. 15. The bars represent concentrations of alkyl sulphate (AS), alkyl ether sulphate (AES) and cocoamidopropyl betaine (CAPB) in sediments at S2 (oxic). The concentrations represent half their respective limit of detection (LOD) with the exception of AS in the 0 – 2cm sample from core 2 used for dating by Cs137 (left panel). 21 . IVL report Fig. 16. The bars represent concentrations of alkyl sulphate (AS), alkyl ether sulphate (AES) and cocoamidopropyl betaine (CAPB) in sediments at T1 (anoxic). The concentrations represent half their respective limit of detection (LOD) with the exception of AS in the 0 – 2cm and 2-4 cm samples from core 2 used for dating by Cs137 (left panel). Fig. 17. The bars represent concentrations of Alkyl sulphate (AS), alkyl ether sulphate(AES) and cocoamidopropyl betaine (CAPB) in sediments at T2 (oxic). The concentrations represent half their respective limit of detection (LOD). 22 . IVL report Discussion Sediment characteristics and redox conditions Lake Mälaren The Cs137-data from core M1 indicate that the sediments always have been bioturbated. The interpretation of the surface sediments as being bioturbated and oxygenated, indicates a low to moderate deposition of dry matter. The degradation of organic matter requires oxygen, thus high deposition of organic matter usually leads to anoxic conditions in the surface sediment (Kiirikki et al., 2006; Reed et al. 2011). The Mn-HCl and Mn-Asc inventories (Fig. 3) are practically identical above the depth of bioturbation (10cm) and reflecting the Mn content of deposited particles such as, refractory Mn-oxides and Iron hydroxyoxides and the oxidation of Mn2+ to MnOOH and MnO2. Below the oxygenated and bioturbated zone, reduction of bioturbated Mn-oxides from the oxic zone occurs reduced Mn species mainly MnCO3, are formed in the anoxic zone. However, as described abover it could also be Mn(IV,III) solid and mixed Mn(II/III)-Fe(II/III)-P solid phases which are preserved in the anoxic environment due to limited supply of either sulphate (Hyacinthe and Van Capellen, 2004) or fresh organic matter (Magen et al., 2011). Considering the high amount of refractory terrestrial organic material that can deposited to the sediments in freshwater lakes either explanation or both in conjunction are possible. The refractory terrestrial organic material is not available for further degradation by the microbes Hence, the difference in the inventories of Mn-Asc and Mn-HCl below the bioturbated zone can be attributed to either preserved Mn oxides or hydrous oxides or precipitated MnCO3. The probably higher annual deposition rates of dry matter at M2 (0.26g/cm2) is consistent with the interpretation of this site being anoxic and not bioturbated since (at least) 1986. This deposition is comparable to what has previously been reported from the surrounding area: 0.14 and 0.20 g /cm2 (Östlund et al., 1998), and 0.23 and 0.29 g /cm2 (Jönsson, 2011). The location of the peak in Mn-Asc concentrations (Fig. 3) is poorly constrained, due to the large interval of that section (0-8cm). The sediment core was anoxic from an ocular inspection and devoid of bioturbation. The distribution of dissolved manganese and the two extracted solid fractions Mn-Asc and Mn-HCl with sediment depth can lead to two different interpretations. The first is that the actual location of the peak in Mn-Asc is probably just below a very thin (some mm) oxidised layer. The almost constant porewater Mn2+ concentrations (Fig. 3) with depth indicates pseudo-equilibrium between dissolved Mn2+ and an authigenic Mn-phase, probably MnCO3 (Berner, 1980). The authigenic Mnphase is formed from the reduction of the Mn-oxides, which is indicated by the decrease of Mn-Asc with sediment depth. However, as espoused above, high concentrations of dissolved organic matter (DOM), produced during the microbial degradation of sediment organic matter, interferes with the nucleation and growth of MnCO3 (Mucci and Edelborn, 1992). Although rhodocrosite super saturation is reached the precipitation is so slow due to inhibition of DOM that pseudo-equilibrium is attained (Berner, 1980). 23 . IVL report However, this interpretation cannot explain the negative difference between Mn-HCl and Mn-Asc. If manganese oxides are reduced to rhodocrosite at depth, then Mn-HCL would be larger than Mn-Asc. Instead an alternative interpretation of the Mn-Asc and Mn-HCl data is that the manganese oxides, hydrous oxides and Fe,Mn-P solid phases are preserved from reduction either due to low amounts of sulphate or organic matter that can easily be degraded (Hyacinthe and Van Cappellen, 2004; Magen et al., 2011). This pool is a part of the HCl-fracation together with rhodocrosite. There is also a high concentration of dissolved Mn2+ which at pH8 is extracted as together with a solid phase as adsorbed or easily exchangeable. This solid phase could be birnessite (-MnO2) which is formed under oxidising conditions, or a another solid Mn-phase which has survived in the anoxic part of the sediment. Birnessite has an increasing adsorption of Mn2+ with increasing pH. From pH2 to pH8 the adsorption of Mn2+ increases from 2 to 7 moles/m2 (*106) or 0.1 to 1.2 moles/kg MnO2 (Appelo and Postma, 1999). For soils, increased solubility of Mn at pH8 and above under reducing conditions have been demonstrated (Kabata-Pendias, 2004). Thus in addition to the increased sorptive capacity of the solid Mn-phase, there could also be an addition of dissolved Mn2+ which could then be adsorbed to the solid Mn-phase, resulting a negative difference between the Mn-HCL and Mn-Asc inventories. Saltsjön The Cs137-data (appendix) do not show a distinct peak at around the year of maximum deposition, 1986, when the Chernobyl accident occurred. Instead there is a weak decrease upwards from a sediment depth of 12-15cm, which is assumed to correspond to 1986/1987. This vertical distribution can reflect either bioturbation meiofauna or resuspension of sediments due to currents (Bradshaw et al., 2006). Considering the lamination and grain size distribution of the core, bioturbation by meiofauna is the likely candidate. The Baltic Sea Meiofauna has the same capacity of vertical mass transport of sediment as macrofauna and are present also in anoxic surface sediments as long as the bottom water is aerated (Bradshaw et al., 2006). Similarly to the anoxic core from Lake Mälaren (M2), below 10 cm the negative difference between Mn-HCl and Mn-Asc at S1 (Fig. 4) could be explained in this way. The organic matter in the sediments in Saltsjön is to a large extent, 35% of Lake Mälaren origin (Jönsson et al., 2005). Thus a there is a significant part of the organic matter in Saltsjön which is of refractory terrestrial origin. The difference between the Mn-Asc and Mn-HCl inventories in the upper could represent the inventory of MnCO3, which precipitates as MnO2 is reduced as soon as it is buried beneath a thin oxidised zone. There is an increase of Mn-Asc towards the surface, which could indicate a redox-boundary somewhere in the top 5 cm of the sediments. Since the sediment core from S1 is considered to recently have been bioturbated (see below) the oxic zone could be a few cm thick at most. One way to explain the vertical overlap of reduced solid species in the Mn-HCl fraction and a redox boundary is to consider the three dimensional distribution of the redox potential. The distribution of the redox potential is determined by the burrows of the bioturbating organisms which leads to both vertical and lateral gradients. Below the redox boundary the sediment at S1 is anoxic. One explanation for the high concentrations of Mn(III, IV) oxides and hydroxides (Mn-Asc) in the anoxic part is that there is an on-going reductive 24 . IVL report dissolution of Mn-oxides in the anoxic part of the sediment but the sediment accumulation rate is high enough and the mixing depth is large enough to make the observed residence time in the anoxic part short enough to preserve the Mn-oxides. On a multi-annual time scale, the upward flux of Mn2+ and the downward flux of Mn-oxides are at steady state. If there would have existed Mn2+ data for S1 we should then have seen an increase in Mn2+ concentrations with sediment depth. The flux of Mn2+ would then exceed the upward and downward diffusive Mn(II,III) fluxes generated by Mn(II,III) re-oxidation and authigenic carbonate formation respectively (Mouret et al., 2009). According to Cs137-activity data (see appendix), its distribution with sediment depth could indicate a recent re-distribution upward from a buried maximum (1986), which could have been caused by bioturbation of macrofauna or meiofauna. By combining the Cs137-data and the Mn-data, this model would imply that there is an on-going bioturbation in the top centimetres at S1 which is consistent with the findings in other previously anoxic areas in Saltsjön (Karlsson et al., 2011). The annual deposition rate of dry matter at S1 (0.21g/cm2) is somewhat lower than the average value for Saltsjön, 0.29 (s=0.11) g/cm2 (Karlsson et al., 2011). Other studies report similar values for the annual deposition rate of dry matter in Saltsjön; 0.20g/cm2 (Östlund et al., 1998) and 0.52;0.40;0.31 g/cm2 (Jönsson, 2011). The combined Mn-data and Cs137-activity data from S2 can be explained by the following model (Mouret et al., 2009). There is an intensive bioturbation at S2, which transport Mn(III, IV) –oxides and hydroxides down to the redox boundary. Throughout the oxic top 4 cm of the sediments, these Mn-oxides are stable. As they reach the anoxic part of the sediment, they become reduced and a peak in porewater Mn2+ is produced. Thus the explanation for the presence Mn-Asc in the anoxic part of the sediment is the same here as for S1. In the case of S2, this explanation is supported by the Mn2+ data, which show a local maximum in the top 4 cm of the sediment core, which is oxic and bioturbated. The increase in porewater Mn2+ indicate that there is no equilibrium between aqueous Mn2+and an authigenic Mn-phase. The negetative difference between Mn-HCl and Mn-Asc in the upper part of the sediment core, could also here be explained by increased solubility of manganese at pH8 under the reducing conditions followed by adsorption solid phase with increasing sorptive capacity at pH 8 compared to pH 2. Torsbyfjärden In the sediment core from station T1, the maximum in the Mn-Asc inventory is in the top 4 cm (Fig. 5), which indicate a redox-boundary somewhere in that interval. There is an increase in the porewater Mn2+ concentration with depth below 10 cm. This could indicate that Mn(III,IV) oxides and hydroxides are being dissolved in the anoxic part of the sediment, which produces an increase in dissolved Mn(II) at depth. The increase in porewater Mn(II) indicates that there is no equilibrium between aqueous Mn(II) and an authigenic Mn-phase. The increase of Mn2+ in the bioturbated oxic part, top 4cm, of the sediment can be explained by this reductive dissolution of Mn-oxides in the anoxic part of the sediment which exceeds the upward and downward diffusive Mn(II,III) fluxes generated by Mn(II,III) re-oxidation and authigenic carbonate formation respectively (Mouret et al., 2009), as can be the case for S1 and S2. 25 . IVL report According to the 137Cs-data (see appendix), the mixing-depth is 10 cm and the average annual dry matter deposition rate is 0.18g/cm2, which is in the range (mean 0.26, s.d. 0.11) of what is found in this and other parts of the Stockholm archipelago (Karlsson et al., 2011). One model which encompass these findings is that bioturbation has recently started in these sediments and thereby transported Mn-oxides down into the anoxic, formerly laminated (i.e. non-bioturbated) sediments. This model is in line with the hypothesis of Karlsson et al. (2011) on the recent bioturbation by Marenzelleria of previously anoxic sediments in the Stockholm Archipelago. At the other station in Torsbyfjärden, T2, the sediment Mn-data (Fig. 5) and 137Cs-activity data from T2 also indicate a bioturbation which buries Mn-oxides in the anoxic part of the sediment. However the inventories of Mn-Asc and Mn-HCl in mole/g are smaller compared to T1. Unfortunately, there are no Mn2+ porewater data to indicate whether there is an equilibrium between aqueous Mn2+ and an authigenic Mn-phase or not. A porewater Mn(II) profile would also facilitate the interpretation of the depth of the redox-boundary. Once again the presence of Mn-Asc in the anoxic part of the sediment can be explained by the same mechanism as for S1, S2 and T2 The 137Cs-activity data (see appendix) from T2 indicate that sediments have always been bioturbated and that the annual deposition rate of dry matter is rather low, 0.11g/cm2 compared to other areas in the Stockholm archipelago, see above. Trends between stations Over all trends for all three pair of stations: Mn-inventories are always higher at anoxic than oxic stations. This relationship can be explained by higher particle deposition rates at the anoxic stations, since all the main factors which control sediment redox conditions, e.g. the deposition flux and reactivity of organic matter, the intensities of bioturbation, and the manganese and iron deposition fluxes, all tend to correlate with sedimentation rate (Tromp et al., 1995). However, it can also be explained by the adverse relationship between particle mixing (caused by bioturbation) and manganese cycling. Particle mixing enhances the transport of particle bound Mn2+ to the sediment-water interface where it is oxidised and returned to the water column ((Wang and van Cappellen, 1996). Without bioturbation, Mn2+ is produced from the reduction of Mn(III,IV) below the rather thin oxic sediment layer caused by the downward diffusion of O2. This thin oxic zone and nitrate in a suboxic zone below it effectively stops the Mn2+ from leaving the sediment since they are oxidised into stable Mn(III,IV) phases, MnOOH and MnO2 (Wang and van Cappellen, 1996; Aller et al., 1998; Mouret et al., 2009). Therefore there exists a negative relationship between maximum concentration of MnO2 in the sediment and particle mixing coefficient (Wang and van Cappellen, 1996). The stations in Lake Mälaren, M1 and M2 have the highest inventories of manganese. This could be explained by both the favourable conditions for precitpitaton of rhodocrosite, due to carbonate content of pore water and preservations of solid Mn(IV,III) phases due to lower concentrations of either sulphate or fresh organic matter, or both. 26 . IVL report Spatial distributions of linear alkylbenzene sulphonates, alkyl sulphates, alkyl ether sulphates and cocoamidopropyl betaine Linear alkylbenzene sulphonates (LAS) has previously been measured in sediments from Lake Mälaren by Kaj et al. (2008) and in sediments from the area Torsbyfjärden by Folke et al. (2003). Kaj et al. (2008) found concentrations in the range 360-1600 ng/g dry matter (dm) in surface sediment sampled in central Stockholm. In surface sediment from Torsbyfjärden, Folke et al. (2003) determined a concentration of 400 ng/kg dm. Concentrations in sediments from Lake Mälaren and from Torsbyfjärden determined in previous studies were thus in the same range as found in the present study. No previous data exist for the area Saltsjön. The spatial distribution in sediments in central Stockholm of nonylphenols (NP), the metabolites of another type of synthetic surfactants, the nonylphenol ethoxylates (NPEOs), show a strong concentration to the discharge points in Saltsjön of the WWTPs in Henriksdal and Bromma (Jönsson, unpublished results). The concentrations in Lake Mälaren and eastern part of Saltsjön, a few km upstream and downstream respectively are significantly lower. An interesting aspect of this spatial distribution of LAS in sediments is that it has been constant even when the consumption of LAS in Sweden was much higher and therefore the loads from the WWTPs, as evident from the higher concentrations of LAS further down in the sediment record (Figs. 6 – 11). This result corroborates the hypothesis of Woldegiorgis (2011) that a return to previous levels of LAS consumption in Sweden would not increase the areas where the benthic fauna would be at risk due to concentrations of LAS in the sediments. The areas at risk would be the same as currently: sediments close to the discharge points of WWTPs where organic matter accumulates. Pronounced horizontal gradients from the discharge of wastewater effluents were also found for LAS in an estuarine environment in Spain (Gonzales-Mazo et al., 1998; Lara-Martin et al., 2006) and in Portugal (Hampel et al., 2009) and in a marine (open sea) environment in Spain (Petrovic et al., 2002). The explanation is the hydrophobicity of LAS which links its environmental fate with that of organic carbon, that is LAS sediments where the particulate organic matter sediments (Leon et al., 2001; Lara-Martin et al., 2006). The high variability of LAS concentrations in Torsbyfjärden, between both oxic and anoxic stations can possibly be explained by some stations receiving effluents from the nearby located WWTP in Käppala on the island Lidingö, whereas the other station reflect the distant load from the city of Stockholm, 20km upstream. 27 . IVL report Table 4. Previous measurements of linear alkylbenzne sulphonates LAS in sediments. Area Stora Essingen, Årstaviken and Riddarfjärden (eastern part of Lake Mälaren, central Stockholm) Fjords of Little Belt, Denmark Concentration (mg/ kg dw) Reference 1.6, 0.36 and 0.53 Kaj et al., 2008 ≤0.1-19 Folke et al., 2003 Torsbyfjärden (Stockholm) and Baltic Proper (open sea north of Gotland) 0.4 and 0.8 German Baltic and North Sea coasts <0.039-0.106 Bester et al., 2001 Danish harbours, Copenhagen area 0.2-28 Nerpin et al., 2005 0.3-8.4 Danish harbours Jensen & Gustavson, 2001 Tagus estuary (Lissabon area, Portugal), 40 stations sampled 2004 0.03-17.76 (July) Hampel et al., 0.09-9.57 2009 (December) Cadiz Bay area (Spain), 5 stations sampled in 2002. Highest conc. close to an untreated waste water discharge. 1.2-67.6 Cadiz Bay area, 11 stations sampled in 2002. Highest conc. same station as Lara-Martín et al., 2006, but half year after WWTP start. 1-10 Lara-Martín et al., 2006 Lara-Martín et al., 2005 Spanish mediterrian and atlantic coasts. Harbours and close to industrial and waste water outflows. 39 samples analysed 19992000. 0.1-238 Petrovic et al., 2002 Bay of Cadiz, Spain,21 samples along transect from municipal discharge point 1.5 - 50 Gonzales-Mazo et al., 1998 The statistical tests (tables 2 and 3) show that the major factor for explaining variations in concentrations is distance from discharge points of WWTPs and not redox conditions of surface sediments. There are only significant differences between the surface concentrations of Saltsjön and Torsbyfjärden for the C11, C12, C13 and sum of homologs C10-C13. This may indicate that C10 is transported longer distances and not deposited to the same extent at the discharge points of the WWTPs due to weaker sorption to sediment particles and having a lower degradation rate in the sediments compared to the longer chain homologs. The capacity of C10 homolog to be transported longer and to be less 28 . IVL report degraded is also supported by the findings in other studies (Leon et al., 2001; Lara-Martin et al., 2006). For the alkyl ether sulphates (AES) as indicated by the parameter sum-AES and the homolog 1-ethoxylate (EO) there is possibly a weak gradient from the discharge points of the WWTPs at S1 compared to both upstream in Lake Mälaren as well as downstream in Torsbyfjärden (Figs. 12 – 17 and table A7). The same type of gradient of decreasing concentrations in sediments with distance from discharge points of wastewater, treated or untreated, has been observed elsewhere (Lara-Martin et al., 2006). However, for the present study the explanation for the much lower concentrations of AES compared to LAS although the use of LAS is lower than AES, is the difference in octanol-water partition coefficients (Kow). The log Kow value for a linear LAS, e.g. 68411-30-3 (11 – 13 carbon atoms in the alkyl chain), is estimated to be 3.32 when calculated as having 11.6 carbon atoms in the alkyl chain (HERA, 2009) For AES with 2.7 (average for household use) ethoxylates the log Kow values are estimated to vary between 0.95 (12 carbon atoms) and 1.9 (14 carbon atoms) depending on carbon chain length (HERA, 2004). Around 95% of all AES used has carbon chain lengths of 12 to 14 carbon atoms(HERA, 2004). There is a small variation in log Kow values depending on the number of ethoxylates in the structure of AES. The average for all AES produced is 2.4 which results in a estimated log Kow values of 1.0 (12 carbon atoms) and 2.0 (14 carbon atoms) depending on carbon chain length (HERA, 2004). Vertical Profiles and aerobic versus anaerobic degradation Vertical concentration profiles of linear alkylbenzene sulfonates (LAS) in sediment have previously been studied by León et al. (2001) and Lara-Martín et al. (2006). León et al. (2001) found a strong decrease in LAS concentration in the upper oxic sediment zone, whereas the decrease in concentration in the deeper anoxic sediment was less steep. The decrease in LAS concentrations with sediment depth and the concurrent increase in sulfophenyl carboxylic acids (SPCs) indicate the possibility of anaerobic degradation of LAS in the sediments. SPCs are key intermediate metabolites in the anaerobic degradation of LAS (Lara-Martin et al., 2007). However, Leon et al. (2001) could not rule out the possibility of the increase in LAS at the sediment surface reflected the increased use of LAS due to an increase in the population generating the urban wastewater effluents. LaraMartín et al. (2006) found a similar profile and also concluded that anaerobic degradations of LAS producing SPCs takes place below the bioturbation depth, where the sediment is anoxic. Indeed, Lara-Martin et al. (2007) could conclude from a laboratory study with anaerobic sediments spiked with LAS, that anaerobic degradation takes place and during this process SPCs are produced. Lara-Martin et al. (2007) estimated a half-life of approximately 90 days for LAS in anaerobic sediments, but significantly higher values, up to several years, expected at concentrations above 20 ppm dw due to microbial toxicity. 29 . IVL report In the sediment cores from anoxic surface sediments in Saltsjön (S1) the peak in LAS (Fig.8) concentrations at 10 – 15cm depth probably reflects a change in the use of LAS. As espoused above, physical reworking of the sediments caused by erosion seems unlikely but bioturbation by meifauna is likely to be present. If the mass transport caused by meiofauna bioturbation would be the dominating process, the LAS concentration profile would mimic that of Cs137 considering the high affinity to particulate organic carbon (Leon et al., 2001; Lara-Martin et al., 2006). To some extent it does follow the curve of Cs137-activity but the decline is much sharper closer to the sediment surface. This could reflect decreased use of LAS in later years but also rapid degradation in a thin oxic layer close to the surface. In the sediments below the peak in LAS concentrations, there could be anaerobic degradation of LAS occurring (c.f. Lara-Martin et al., 2007) since this period, i.e. pre-1986, would correspond to higher depositions of LAS. A natural starting point for discussing the vertical distribution of LAS in the sediments of the study area is to start close to the discharge points of the wastewater effluents since this is the point where changes in the use of LAS will be most evident. In the oxic surface sediments in Saltsjön (S2), the peak in LAS concentrations are in the region of 2 to 10 cm of sediment depth (Fig.9). In this region poor correlation between LAS concentration and Cs137-activity profiles could be attributed to discrimination of inorganic and organic matter in mass transport processes (bioturbation) by meiofauna and macrofauna. In this case, Monoporeia affinis, which was observed in the overlying water of some of the cores, cause both upward and downward transport which could distribute compounds bound to organic matter bound differently than more weakly adsorbed compounds to mineral surfaces (Bradshaw et al., 2006). One example of the former could be LAS and one of the latter could be Cs+. Below the zone of bioturbation. Considering the bioturbation depth of Momoporeia affinis, which is would be expected to be less than 10cm (Bradshaw et al., 2006), the position of the peak in LAS concentrations at S1 probably also reflects a dccrease in the use of LAS during the last decade or so. The decreasing concentrations at S1 (Fig. 8) probably also here reflects anaerobic degradation of LAS. Using the interpretations of the sediment profiles of LAS from stations S1 and S2 in Saltsjön (Fig. 8 and 9) , close to the discharge points of the WWTPs as indicatiors of LAS use in the Stockholm region, the same processes can be seen in sediments from Eastern Lake Mälaren (Fig. 6 and 7). At the anoxic site (M2), the sediments would be without any pronounced redistribution of LAS due to bioturbation, an the vertical profile reflects a decreasing use of LAS during the recent decade(s). At the oxic site (M1), bitoturbation by meiofauna and macrofauna causes the typical vertical profile with a maximum just beneath the sediment surface (Berner, 1980; Bradshaw et al., 2006), while the peak at 4-6 cm sediment depths reflects an earlier higher deposition of and use of LAS. The vertical profiles of LAS in Torbyfjärden, T2 (Fig. 10 and 11) are more difficult to interpret since there are large relative variations between the cores at both sites and the vertical resolution is in some instances very poor. The core with the highest concentration of LAS, and hence highest deposition of organic particles, at the anoxic site (T1) seems to clearly indicate on going bioturbation while the other one does not. Likewise the core with the highest concentrations of LAS and deposition of organic matter at the oxic site (T2) can be interpreted as bioturbation occurs in the top 6cm. 30 . IVL report Ecotoxicity The toxicity of linear alkylbenzene sulphonate (LAS) towards freshwater and marine benthic species has been studied by several authors (Tables 5 and 6). Reviews of available data show that acute toxicity is greater for individual LAS homologues with longer alkyl chain lengths and that biodegradation intermediates are significantly less toxic than the parent LAS with L/EC50 values >1000 mg/L for fish and crustacean (Daphnia.magna) (UNEP 2005). Based on chronic toxicity data for three freshwater species of different taxonomic groups, Comber et al. (2006) suggested a PNEC of 8.1 mg/kg dw, using an assessment factor of 10. For marine sediments Hampel et al. (2007) derived a PNEC of 4.9 mg/kg dw using an assessment factor of 10 based on chronic ecotoxicity data for two mollusc and one fish species. The endpoint studied resulting in the lowest value was lethality and that the study duration was relatively short (9 days). Further, this PNEC-value is in the same range as concentrations shown to cause acute effects on marine algae (Moreno-Garrido et al. 2003; Moreno-Garrido et al. 2007). The PNECs derived are in the same range as site-specific sediment quality values (SQVs)for the Gulf of Cádiz, derived by linking chemical measurements and biological effects (sediment toxicity results for the clam Ruditapes philippinarum and the amphipod Microdeutopus gryllotalpa;; DelValls et al., 2002). In this study LAS, Pb and Ag were grouped together with the biological effects. For LAS the analysis resulted in the SQV “Not polluted” (low or minimal biological effects) of <2.6 mg/kg dw, and the SQV “Highly polluted” (high biological effects) for concentrations >8.7 mg/kg dw. Table 5. Chronic toxicity of sediments spiked with LAS (mg/kg dw) towards benthic freshwater species.mg/kg dw Species Duration Effect Value Lumbriculus variegatus 28 days NOEC Endpoint 81 survival/reproduction Comment Reference 1.7% OC Comber et al., 2006 Caenorhabditis elegans 72 h NOEC 100 egg production 2% OC Chironomus riparius 10 days NOEC 348 head capsule length C12-LAS, 3.2% OC Comber et al., 2006 Mäenpää & Kukkonen 2006 Chironomus riparius 24 days NOEC 319 emergence 4.2% OC Pittinger et al., 1989 Table 6. Acute and chronic toxicity of sediments spiked with LAS (mg/kg dw) towards benthic marine species. Acute Species Duration Effect Value Hydrobia ulvae 96 h LC50 140.65 mortality Hydrobia ulvae 96 h LC50 101.77 mortality Solea senegalensis Cylindrotheca closterium Cylindrotheca closterium Phaeodactylum tricornutum 96 h LC50 2179.68 mortality 72 h ErC50 17 growth rate 72 h 4.18 growth rate Moreno-Garrido et al., 2003 72 h EbC50 63% inhib. 4.77 growth rate Moreno-Garrido et al., 2007 Corophium volutator 5 days LC50 295 mortality 31 Endpoint Comment Reference 0.618% OC Hampel et al., 2007 Hampel et al., 2009 0.618% OC Hampel et al., 2007 0.59% OC Mauffret et al., 2010b C12-LAS, Rico-Rico et al., 2009 . IVL report 1.38% OC Chronic C12-LAS, 0.06% OC Rico-Rico et al., 2009 0.618% OC Hampel et al., 2007 0.59% OC Mauffret et al., 2010a 48.82 mortality 0.618% OC Hampel et al., 2007 362.99 mortality 0.618% OC Hampel et al., 2007 Corophium volutator Ruditapes philippinarum 5 days LC50 162 mortality 30 days LC10 560.52 mortality Hydrobia ulvae 10 days LC10 124 mortality Hydrobia ulvae 9 days LC10 Solea senegalesis 30 days LC10 Solea senegalesis 30 days NOEC 92.29 mortality Hampel et al., 2008 Solea senegalesis 30 days LOEC 222.66 mortality Hampel et al., 2008 The concentration in the sediments in Lake Mälaren and Torsbyfjärden were considerably lower than in Saltsjön, which indicate that a rapid sedimentation of LAS occurs near the source, the WWTP. Exposure to benthic organisms is mainly relevant in oxic sediments, provided that anoxic sediment does not become oxygenated. In Saltsjön the concentrations in the surface, 3.9 – 7.9 mg/kg dw, was within the range of the PNEC suggested by Hampel et al. (2007) but below the PNEC suggested by Comber et al. (2006). The highest risk ratios (measured concentration/PNEC) for oxic sediments gives values in the range of 1.0 – 1.4 in the surface and a value up to 2.3 in the deeper layers. The highest risk ratios in the anoxic sediments range between 1.5 - 2.5 in the surface and 8.9 in the deeper sections. Thus, the results indicate that the presence of LAS in sediments in the area Saltsjön may have a negative impact on benthic organisms today in the local area in the immediatie vicinity of the discharge points of the two WWTPs of Stockholm city, Henriksdalsverket and Brommaverket.The exact size of this area is uknown but is probably of a similar size to that of nonylphenols, about 1 km2 (A. Jönsson unpublished results). As pointed out by Woldegiorgis (2011), sediment toxicity data for the other anionic surfactants alkyl sulphates (AS) and alkyl ether sulphates (AES) and amphoteric cocoamidopropyl betaine (CAPB), are very scarce to non-existent but should be considered to have similar toxicity compared to LAS based on toxicity data for water. In the case of AS it could also be considered to have higher ecotoxicity for both water, sediment and soil based on the limited data available (Fraunhofer Institut, 2003). The proposed sediment predicted no effect concentrations (PNEC) in g/g dry weight for AS varies depending on chain length: 0.0963 (12 carbon atoms), 0.0406 (13 carbon atoms), 0.0186 (14 carbon atoms), 0.22 (15 carbon atoms), 0,468 (16 carbon atoms) and 8.4 (18 carbon atoms). The three shortest chain lengths (C12 – C14) make up about 61% of the AS used in EU, Norway and Switzerland, whereas chain lengths of 15, 16 and 18 carbon atoms make up the remaining 39%. Thus, the concentrations found in the surface sediments in Saltsjön at S1 (Fig. 14) and Torsbyfjärden at T1 (Fig. 16) indicate that there is a risk for local negative environmental effects. The risk ratios can be as high as 0.322/0.0186 = 17 at T1 and 0.118/0.0186=6 at S1. These risk ratios are higher than the ones for LAS identified in this study. Thus on a local scale, the environmental threat to the benthic fauna can be larger from the surfactant AS than it is from LAS. The findings of this study corroborate one of the hypotheses of Woldegiorigis (2011): 32 . IVL report The environmental risk for the benthic fauna on a local scale is equal or greater from other surfactants, in this case AS, than that of LAS. This is true even when considering the higher previous loads of LAS as indicated in the sediment record. Conclusions The main factor determining sediment concentrations of linear alkylbenzne sulphonate (LAS) in the Stockholm area is where sedimentation of organic carbon occurs and distance from the point of waste water discharge. The redox environment (oxic/anoxic) of the surface sediments does not influence the distribution of LAS on a kilometre wide scale. There seems to be anaerobic degradation of LAS occurring in the sediment of, Lake Mälaren and inner Stockholm archipelago (Saltsjön and Torsbyfjärden). There are potential local environmental threats to the benthic fauna due to the concentrations of LAS and alkyl sulphates (AS) in the sediments where the two WWTPs of the city of Stockholm discharge their treated waste water. In the case of AS, this threat also exists also about 20km further downstream in Torsbyfjärden, close to the discharge point of the WWTP Käppala. The study corroborates the two main hypotheses of Woldegiorgis (2011): o Sediment concentrations of LAS may pose an environmental risk for the benthic fauna only on a local scale close to the discharge points of WWTPs where organic matter accumulates and this will not change after a return to previous higher levels of LAS consumption o If LAS is replaced by another surfactant the environmental threat to the benthic fauna on this local scale will be shifted from LAS to the other surfactant since the overall use of surfactants will not decrease. In the present case, this threat comes from AS 33 . IVL report References Acmite, 2010. Market report – World surfactant market. Acmite Market Intelligence, Ratingen, Germany. Appelo, C.A.J., Postma, D., 1999. A consisten model for surface complexation of Birnessite (-MnO2) and its application to a column experiment. Geochimica et Cosmochimica acta. 63: 3039-3048. Aller, R.C., Hall, P. O. J., Rude, P.D., Aller, J.Y. 1998. Biogeochemical heterogeneity and suboxic diagenesis in hemipelagic sediments of the Panama Basin. Deep-Sea Research I 45:133-165. Bester, K., Theobald, N., Schröder, H. 2001. Nonylphenol-ethoxylates, linear alkylbenzenesulfonates (LAS) and bis (4-chlorophenyl)-sulfone in the German Bight of the North Sea. Chemosphere, 45: 817-826. Berner, R.A. 1980. Early diagenesis – A theoretical approach. Princeton University Press, Princeton N. J. Bradshaw, C., Kumblad, L., Fagrell, A. The use of tracers to evaluate the importance of bioturbation in remobilising contaminants in Baltic sediments. Estuarine, Coastal and Shelf Science. 66: 123-134. Calvert, S.E., Pedersen, T.F., Karlin, R.E. 2001. Geochemical and isotopic evidence for post-glacial palaeoceanographic changes in Saanich Inlet, British Columbia. Marine Geology. 174: 287-305. 34 . IVL report Canfield, D.E., Thamdrup, B., Hansen, J. W., 1993. The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulphate reduction. Geochimica et Cosmochimica acta. 57: 3867-3883. Ceresana Research, 2012. Market Study: Surfactants. Ceresana, Konstanz, Germany. Fischer, T.B., Heaney, P.J., Jang, J-H., Ross, D.E., Brantley, S.L., Post, J. E., Tien, M. 2008. Continuous time-resolved X-ray diffraction of the biocatalyzed reduction of Mn oxide. American Mineralogist. 93: 1929–1932. Comber, S. D. W., Conrad, A. U., Höss, S., Webb, S., Marshall, S. 2006. Chronic toxicity of sediment-associated linear alkylbenzene sulphonates (LAS) to freshwater benthic organisms. Environ. Pollut. 144: 661-668. DelValls, T.Á., Forja, J.M., Gómez-Parra, A. 2002. Seasonality of contamination, toxicity, and quality values in sediments from littoral ecosystems in the Gulf of Cádiz (SW Spain). Chemosphere, 46: 1033-1043. Folke, J., Cassani, G., Ferrer, J., Lopez, I., Karlsson, M., Willumsen, B. 2003. Linear alkylbenzene sulphonates, branched dodecylbenzene sulphonates and soap analysed in marine sediments from the Baltic Proper and Little Belt. Tenside Surf. Det. 40: 17-24. Fraunhofer Institut, 2003. Anaerobic biodegradation of detergent surfactants – final report. Fraunhofer Institut fur Umwelt-, Sicherheits- und Energietechnik,. Oberhausen Germany. Gonzalez-Mazo, E., Forja, J. M., Gomez-Parra, A. 1998. Fate and distribution of linear Alkylbenzene sulfonates in the litoral environment. Environ. Sci. Technol. 32:16361641. Hampel, M., Gonzáles-Mazo, E., Vale, C., Blasco, J. 2007. Derivation of predicted no effect concentrations (PNEC) for marine environmental risk assessment: Application of different approaches to the model contaminant Linear Alkylbenzene Sulphonates (LAS) in a site-specific environment. Environ. Int. 33: 486-491. Hampel, M., Ortiz-Delgado, J.B., Sarasquete, C., Blasco, J. 2008. Effects of sediment sorbed linear alkylbenzene sulphonate on juveniles of the Senegal sole, Solea senegalensis: Toxicity and histological indicators. Histol. Histopathol. 23: 87:100. Hampel, Canário, J., Branco, V., Vale, C., Blasco, J. 2008b. Environmental levels of linear alkylbenzene sulfonates (LAS) insediments from the Tagus estuary (Portugal): environmental implications. Environ. Monit. Assess. DOI 10.1007/s10661-008-0190-0 Hampel, M., Moreno-Garrido, I., Gonzáles-Mazo, E., Blasco, J. 2009. Suitability of the marine prosobranch snail Hydrobia ulvae for sediment toxicity assessment: A case study with the anionic surfactant linear alkylbenzene sulphonate (LAS). Ecotox. Environ. Safety, 72: 1303-1308. Hyacinthe, C., Anschutz, P., Carbonel, P., Jouanneau, J.-M., Jorissen, F.J. 2001. Early diagenetic processes in the muddy sediments of the Bay of Biscay. Marine Geology 177: 111-128. 35 . IVL report Hyacinthe, C., Van Cappellen, P. 2004. An authigenic iron phosphate phase in estuarine sediments: composition, formation and chemical reactivity. Marine Chemistry 91: 227– 251. Human & Environmental Risk Assessments (HERA) on ingredients of European household cleaning products – Alkyl Sulphates (AS) Environmental Risk Assessment. 2002. Human & Environmental Risk Assessments (HERA) on ingredients of European household cleaning products – Alcohol Ethoxysulphates (AES) Environmental Risk Assessment. 2004. Human & Environmental Risk Assessments (HERA) on ingredients of European household cleaning products – Linear Alkylbenzene Sulphonate (LAS) Environmental Risk Assessment. 2009. Jensen, A., Gustavson, G. 2001. Havnesedimenters indhold af miljøfremmede organiske forbindelser. Kortlægning af nuværende og fremtidige behov for klapning og deponering. Miljøprojekt Nr. 627. Miljøstyrelsen, Miljø- og Energiministeriet. Jönsson, A., Lindström, M., Carman, R., Mört, C-M, Meili, M and Ö Gustafsson. 2005. Evaluation of the Stockholm Archipelago Northwestern Baltic Sea Proper, as a trap for freshwaterrunoff organic carbon. Journal of Marine Systems, 56: 167 – 178. Jönsson, A. 2011. Ni, Cu, Zn, Cd and Pb in sediments in the city-centre of Stockholm, Sweden Origins, deposition rates and bio-availability. IVL-B2013. Kabata-Pendias, A. 2001. Trace elements in soils and plants, 3rd ed. CRC Press, Boca Raton. Kaj, L., Lilja, K., Remberger, M., Allard, A-S., Dusan, B., Brorström-Lundén, E. 2008. Results from the Swedish National Screening Programme 2007. Subreport 4: Linear alkyl benzene sulfonate (LAS). IVL Report B1808. October 2008. Karlsson, M., Malmaeus, M., Rydin, E., Jonsson, P. 2010. Bottenundersökningar i Upplands, Stockholms, Södermanlands och Östergötlands skärgårdar .2008-2009. IVL, report B1928, in Swedish. KEMI, 2012a. Flow analyses for chemical substances: Linear alkylbenzene sulfonates. http://apps.kemi.se/flodessok/floden/flodessok.cfm?sokval KEMI, 2012b. Flow analyses for chemical substances: Sodium dodecyl sulphate. http://apps.kemi.se/flodessok/floden/flodessok.cfm?sokval KEMI, 2012c. Flow analyses for chemical substances: sodium dodecyl eter sulphate. http://apps.kemi.se/flodessok/floden/flodessok.cfm?sokval KEMI, 2012d. Flow analyses for chemical substances: Coco amidopropyl betaine. http://apps.kemi.se/flodessok/floden/flodessok.cfm?sokval Kiirikki, M., Lehtoranta, J., Inkala, A., Pitkänen, H., Hietanen, S., Hall, P.O.J., Tengberg, A., Koponen, J., Sarkkula, J. A simple sediment process description suitable for 3Decosystem modelling — Development and testing in the Gulf of Finland. Journal of Marine Systems 61: 55–66. 36 . IVL report Lara-Martín, P.A., Gómez-Parra, A., González-Mazo, E. 2005. Determination and distribution of alkyl ethoxylates and linear alkylbenzene sulfonates in coastal marine sediments from the bay of Cadiz (southwest of Spain). Env. Toxicol. Chem. 24:21962202. Lara-Martín, P. A., Petrovic, M., Gómez-Parra, A., Barceló, D., González-Mazo, E. 2006. Presence of surfactants and their degradation intermediates in sediment cores and grabs from the Cadiz Bay area. Environ. Pollut. 144: 483-491. Lara-Martín, P. A., Gómez-Parra, A., Köchling, T., Sanz, J. L., Amils, R., González-Mazo, E. 2007. Anaerobic degradation of linear alkylbenzene sulfonates in coastal marine sediments. Environ. Sci. Technol. 41: 3571-3579. León, V.M., Gonzáles-Mazo, E., Pajares, J.M.F., Gómez-Parra, A. 2001. Vertical distribution profiles of linear alkylbenzene sulfonates and their long-chain intermediate degradation products in coastal marine sediments. Environ. Toxicol. Chem. 20: 21712178. Mäenpää, K., Kukkonen, J.V.K. 2006. Bioaccumulation and toxicity of 4-nonylphenol (4NP) and 4-(2-dodecyl)-benzene sulfonate (LAS) in Lumbriculus variegatus (Oligochaeta) and Chironomus riparius (Insecta). Aquat. Toxicol. 77: 329-338. Magen, C., Mucci, A., Sundby, B. Reduction rates of sedimentary Mn and Fe Oxides: An incubation experiment with Arctic Ocean sediments. Aquat Geochem. 17: 629–643. Mauffret, A., Moreno-Garrido, I., Blasco, J. 2010a. The use of marine benthic diatoms in a growth inhibition test with spiked whole-sediment. Ecotox. Environ. Safety, 73: 262269. Mauffret, A., Temara, A., Blasco, J. 2010b. Exposure of the marine deposit feeder Hydrobia ulvae to sediment spiked with LAS homologs. Water Res. 44: 2831-2840. Moreno-Garrido, I., Hampel, M., Lubián, L.M., Blasco, J. 2003. Marine benthic microalgae Cylindrotheca closterium (Ehremberg) Lewin and Reimann (Bacillariophyceae) as a tool for measuring toxicity of linear alkylbenzene sulfonate in sediments. Bull. Environ. Contam. Toxicol. 70: 242-247. Moreno-Garido, I., Lubián, L.M., Blasco, J. 2007. Sediment toxicity tests involving immobilized microalgae (Phaeodactylum tricornutum Bohlin). Environ. Int. 33: 481-495. Morford, J. L., Russel, A.D., Emerson, S. 2001. Trace metal evidence for changes in the redox environment associatied with the transition from terrigenous clay to diatomaceous sediment, Saanich Inlet, BC. Marine geology. 174: 355-369. Mouret, A., Anschutz, P., Lecroart, P., Chaillou, G., Hyacinthe, C., Deborde, J., Jorissen, F. J., Deflandre, B., Schmidt, S., Jouanneau, J-M., 2009.Benthic geochemistry of manganese in the Bay of Biscay, and sediment mass accumulation rate. Geo-Mar. Lett. 29:133-149. Mucci, A., Edelborn, H.M. 1992. Influence of an organic-poor landslide on the early diagenesis of iron and manganese in a coastal marine sediment. Geochimica et Cosmochimica Acta, 56: 3909 – 3921. 37 . IVL report Naturskyddsföreningen (2006) Kemiska Produkter.Version 2006:4 Nordic Ecolabelling (2012) Laundry detergents and stain removers. Version 7.1. 15 December 2011-31 December 2015. Nerpin, L., Nordell, O., Burgdorf Nielsen, J., Hein, M., Carlsson, C., Bjerre, F., BrønsHansen, J. 2005. Miljögifter i Öresund, en översikt. Öresundsvattensamanbetet. www.oresundvand.dk Petrovic, M., Fernández-Alba, A.R., Borrull, F., Marce, R.M., González-Mazo, E., Barceló, D. 2002. Occurence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Env. Toxicol. Chem. 21: 37-46. Pittinger, C.A., Woltering, D.M., Masters, J.A. 1989. Bioavailability of sediment-sorbed and aqueous surfactants to Chironomus riparius (midge). Environ. Toxicol. Chem. 8: 10231033. Reed, D.C., Slomp, C.P., Gustafsson, B. G. 2011. Sedimentary phosphorus dynamics and the evolution of bottom-water hypoxia: A coupled benthic–pelagic model of a coastal system. Limnol. Oceanogr., 56(3): 1075–1092 Rico-Rico, Á., Temara, A., Hermens, J.L.M. 2009. Equilibrium partitioning theory to predict the sediment toxicity of the anionic surfactant C12-2-LAS to Corophium volutator. Environ. Pollut. 157: 575-581. SSNC, 1999. Hushållskemikalier i förändring. En statistisk jämförelse mellan åren 1988 och 1996. Svenska Naturskyddsföreningen 1999. In Swedish. Thiessen, O., Schmidt, M., Theilen, F., Schmitt, M., Klein, G. 2006. Methane formation and distribution of acoustic turbidity in organic-rich sediments in the Arkona Basin, Baltic Sea. Continental Shelf Research, 26: 2469-2483. Tromp, T., K., Van Cappellen, P., Key, R.M., 1995. A global model for the early diagenesis of organic carbonand organic phosphorus in marine sediments. Geochimica et Cosmochimica Acta, 59 (7): 1259 – 1284. Turner, A., Millward, G.E. Particle Dynamics and Trace Metal Reactivity in Estuarine Plumes. Estuarine, Coastal and Shelf Science, 50: 761–774. Wang, Y., van Cappellen, P. 1996. A multicomponent reactive transport model of early diagenesis: Application to redox cycling in coastal marine sediments. Geochimica et Cosmochimica Acta, 60 (16): 2993-3014. Whiticar, M. J. 1999. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chemical Geology, 161: 291-314. Woldegiorgis, 2011. Environmental risk and hazard assessment of LAS compared to other anionic surfactants- Scenario-based waiving of LAS in a Swedish perspective. . IVL, report Bxxxx. In English. Östlund, P., Sternbeck, J., Brorström-Lundén, E. 1998. Metaller, PAH, PCB och totalkolväten i sediment runt Stockholm – flöden och halter. IVL, report B1297. In Swedish. 38 . IVL report Appendix Table A1. Cs137-activity data. Lokal M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 M1 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 21-22 22-23 23-24 24-25 25-26 26-27 27-28 28-29 CPM 20.7 25.7 30.7 23.9 17.6 13.9 12.5 13.5 16.3 18.0 12.8 14.1 13.2 13.8 11.5 8.8 8.9 7.1 8.8 15.8 22.3 22.7 19.4 23.1 Lokal M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 M2 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 21-22 22-23 23-24 24-25 25-26 26-27 27-28 28-29 29-30 30-31 31-32 32-33 33-34 34-35 35-36 36-37 37-38 38-39 39-40 40-41 41-42 42-43 43-44 44-45 45-46 46-47 47-48 48-49 CPM 16.9 19.3 20.8 21.0 31.6 29.4 31.4 29.9 42.3 29.0 25.5 18.6 16.4 13.8 10.9 15.7 9.7 9.5 10.2 12.2 11.0 13.3 12.0 10.0 14.0 14.3 15.7 12.2 13.9 14.3 16.4 13.4 15.9 13.9 17.5 17.5 14.1 14.4 12.9 14.4 14.2 11.5 13.7 11.9 Lokal S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 S1 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 CPM 23.9 26.7 26.6 32.0 25.9 26.8 27.6 36.4 36.2 31.4 19.9 15.1 12.9 9.2 10.9 14.6 Lokal S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 S2 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 21-22 22-23 23-24 24-25 25-26 26-27 27-28 28-29 29-30 30-31 31-32 CPM 18.9 14.5 13.9 15.9 17.3 11.0 7.0 9.0 8.9 8.2 6.3 5.5 6.6 8.0 9.4 6.9 6.7 7.9 8.1 10.2 8.1 7.9 10.9 9.2 12.7 12.1 3.9 Lokal T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 T1 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 21-22 CPM 28.9 36.4 43.6 46.6 45.4 18.0 22.6 26.3 20.1 25.3 26.0 18.5 20.3 20.4 14.6 28.5 32.5 Lokal T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 T2 Skikt (cm) 0-2 2-4 4-6 6-8 8-10 10-11 11-12 12-13 13-14 14-15 15-16 16-17 17-18 18-19 19-20 20-21 21-22 22-23 CPM 40.0 43.9 30.4 20.3 13.5 10.7 11.2 10.3 8.6 9.5 10.6 8.5 8.2 8.0 10.2 12.8 13.1 16.9 Table A2 Mn-speciation data: Mn-Asc and Mn-HCl concentrations and inventories in Lake Mälaren sediment cores Core depth Mn-Asc Mn-HCl (mmol/g1) (mmol/g1) Mn2+ (mM) % dm 39 I-Asc I-HCl (mmol/cm2) (mmol/cm2) . IVL report (cm) M1:0-1 49 46 16 0.84 21 20 M1:1-6 41 39 19 0.81 104 99 M1:6-11 25 28 24 0.76 79 86 M1:11-16 27 40 20 0.80 73 106 M2:0-5 127 36 20 10 0.90 176 50 25 20 16 0.84 M2:5-10 52 M2:10-15 64 30 21 18 0.82 157 74 M2:15-20 73 23 22 20 0.80 193 61 M2:20-28 51 30 21 20 0.80 214 125 Table A3. Mn-speciation data: Mn-Asc and Mn-HCl concentrations and inventories in Saltsjön sediment cores Core Mn-Asc Mn-HCl (mmol/g) (mmol/g) Mn2+ (mM) %ts 40 I-Asc (mmol/cm2) I-Hcl (mmol/cm2) . IVL report depth (cm) S2: 0-2 12 10 12 53 0.47 35 28 S2:2-4 24 13 19 56 0.44 70 37 S2:4-6 11 14 8.2 34 0.66 20 25 S2:6-11 11 19 5.9 24 0.76 34 58 S2:11-16 9 17 4.9 28 0.72 32 62 S2:16-21 11 20 7.8 21 0.79 31 54 S2:21-26 9 20 9.8 26 0.74 31 68 S1:0-5 12 16 14 0.86 22 30 S1:5-10 8.4 18 20 0.80 23 48 S1:10-15 30 24 14 0.86 55 44 S1:15-20 31 24 12 0.88 51 39 S1:20-25 29 23 11 0.89 45 34 S1:25-30 39 28 13 0.87 66 48 41 . IVL report Table A4. Mn-speciation data: Mn-Asc and Mn-HCl concentrations and inventories in Torsbyfjärden sediment cores Core depth Mn-Asc (mmol/g) Mn-HCl Mn2+ (mmol/g) (mM) %ts I-Asc I-Hcl 2 (mmol/cm ) (mmol/cm2) (cm) T2: 0-5 13 19 17 0.83 30 44 T2: 5-10 13 23 19 0.81 33 57 T2:10-15 16 21 15 0.85 32 42 T2:15-20 20 27 16 0.84 41 56 T2:20-25 21 29 17 0.83 47 67 T1:0-4 40 44 26 17 0.83 71 78 T1:4-9 17 34 19 17 0.83 40 79 T1:9-14 9.3 37 22 17 0.83 21 82 T1:14-19 32 43 33 19 0.81 82 109 T1:19-24 37 38 45 20 0.80 98 101 T1:24-29 29 37 57 21 0.79 80 103 T1:29-34 33 38 65 22 0.79 93 108 42 . IVL report Appendix A5. Measured concentration of the different homologs of linear alkyl benzene sulfonate (LAS) as a function of sediment depth and oxic condition. Sample id Sampling site Sedimentcor e Index MnM1 1521 CsM1 0-2 CsM1 2-4 CsM1 4-6 MnM2 1825 MnM2 1318 MnM2 8-13 CsM2 0-4 CsM2 8-12 CsM2 12-17 Mälaren Core 1 Mälaren Mälaren Mälaren Core 2 Core 2 Core 2 Mälaren Core 1 Mälaren Core 1 Mälaren Mälaren Mälaren Mälaren Torsbyfjärde n Torsbyfjärde MnT1 20-30 n Torsbyfjärde CsT1 0-2 n Torsbyfjärde CsT1 2-14 n MnT1 30-34 Torsbyfjärde n Torsbyfjärde MnT2 15-20 n Torsbyfjärde CsT2 0-2 n Torsbyfjärde CsT2 2-6 n MnT2 20-25 Core 1 Core 2 Core 2 Core 2 Environmen t Oxic/Anoxi c Dept h 10 Carbons Concentration of LAS 11 12 13 Carbons Carbons Carbons cm ng/g dw ng/g dw ng/g dw ng/g dw ng/g dw oxic 0-6 19 78 131 217 444 0-2 2-4 4-6 3.2 1.7 3.1 20 15 26 35 24 54 51 41 74 109 82 157 0-7 21 56 57 85 219 7-12 6.8 38 58 90 193 12-17 0-4 8-12 12-17 10 2.9 3.1 6.5 51 16 30 55 91 27 53 113 139 54 85 182 291 100 170 357 oxic oxic oxic Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic ΣHomolog s Core 1 Anoxic 0-4 33 90 82 64 270 Core 1 Anoxic 4-14 5.3 26 44 50 127 Core 2 Anoxic 0-2 0.5 <2.2 <5.9 <7.9 <11 Core 2 Anoxic 2-14 <0.4 3.3 <5.9 <7.9 <11 Core 1 Oxic 0-5 2.1 9.9 16 20 48 Core 1 Oxic 5-10 4.8 19 40 26 89 Core 2 Oxic 0-2 55 212 324 429 1020 Core 2 Oxic 2-6 4.5 16 26 24 71 MnS1 25-30 MnS1 20-25 MnS1 10-15 CsS1 0-2 CsS1 6-8 CsS1 12-15 CsS1 15-18 CsS1 18-21 Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Core 2 Core 2 Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic 0-5 5-10 15-20 0-2 6-8 12-15 15-18 18-21 46 204 2723 121 1000 1013 480 1000 622 1592 15299 1699 10599 9432 3566 5499 2147 4264 10797 4164 8630 8030 6230 4530 4229 8296 14696 6096 11729 10996 8229 5396 7045 14356 43515 12080 31958 29472 18505 16425 MnS2 24-26 MnS2 20-24 MnS2 15-20 CsS2 0-2 CsS2 2-6 CsS2 6-10 CsS2 10-12 CsS2 14-16 Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Core 2 Core 2 Oxic Oxic Oxic Oxic Oxic Oxic Oxic Oxic 0-2 2-6 6-11 0-2 2-6 6-10 10-12 14-16 14 33 86 15 129 95 76 63 324 622 1062 244 1212 676 489 306 1934 2534 3430 1607 3497 2127 1814 1567 1673 2739 3729 5996 6496 4629 3463 4429 3944 5929 8308 7862 11335 7527 5841 6365 *Smaller than (<) values refers to lowest detection limit (LOD= S/N). 43 . IVL report Appendix A6. Measured concentration of alkyl sulphate (AS) and cocoamidopropyl betaine (CAPB) as a function of sediment depth and oxic condition. Sample id Sampling site Mälaren Mälaren Mälaren Mälaren Sedimentcore Index Core 1 Core 2 Core 2 Core 2 Environment Oxic/Anoxic Anoxic Anoxic Anoxic Anoxic Depth cm 0-6 0-2 2-4 4-6 MnM1 15-21 CsM1 0-2 CsM1 2-4 CsM1 4-6 MnM2 18-25 MnM2 13-18 MnM2 8-13 CsM2 0-4 CsM2 8-12 CsM2 12-17 Mälaren Mälaren Mälaren Mälaren Mälaren Mälaren Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Oxic Oxic Oxic Oxic Oxic Oxic 0-7 7-12 12-17 0-4 8-12 12-17 <22 <22 <22 25 <22 <22 <11 <11 <11 <11 <11 <11 MnT1 30-34 MnT1 25-30 MnT1 20-25 CsT1 0-2 CsT1 2-4 CsT1 4-8 CsT1 8-14 Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Core 2 Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic 0-4 4-9 9-14 0-2 2-4 4-8 8-14 <22 <22 <22 94 322 <22 <22 <11 <11 <11 <11 <11 <11 <11 MnT2 20-25 CsT2 0-2 CsT2 2-4 CsT2 4-6 Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Core 1 Core 2 Core 2 Core 2 Oxic Oxic Oxic Oxic 0-5 0-2 2-4 4-6 <22 <22 <22 <22 <11 <11 <11 <11 MnS1 25-30 MnS1 20-25 MnS1 10-15 CsS1 0-2 CsS1 6-8 CsS1 12-15 Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic 0-5 5-10 10-15 0-2 6-8 12-15 118 37 66 70 42 22 <11 <11 <11 <11 <11 <11 MnS2 24-26 CsS2 0-2 Saltsjön Saltsjön Core 1 Core 2 Oxic Oxic 0-2 0-2 <22 32 <11 <11 *Smaller than (<) values refers to lowest detection limit (LOD= S/N). 44 Concentration of AS ng/g dw <22 26 <22 <22 Concentration of CAPB ng/g dw <11 <11 <11 <11 . IVL report Appendix A7. Measured concentration of the different homologs of alkyl ether sulphate (AES) as a function of sediment depth and oxic condition. Concentration of AES Environment Depth 1 Ethoxylate 2 Ethoxylates 3 Ethoxylates 4 Ethoxylates ΣHomologs Oxic/Anoxic cm ng/g dw ng/g dw ng/g dw ng/g dw ng/g dw Anoxic 0-6 <20 <26 <12 <4.5 <62 Anoxic 0-2 <20 <26 <12 4.8 <62 Anoxic 2-4 <20 <26 <12 <4.5 <62 Anoxic 4-6 <20 <26 <12 <4.5 <62 Sample id Sampling site MnM1 15-21 CsM1 0-2 CsM1 2-4 CsM1 4-6 Mälaren Mälaren Mälaren Mälaren Sedimentcore Index Core 1 Core 2 Core 2 Core 2 MnM2 18-25 MnM2 13-18 MnM2 8-13 CsM2 0-4 CsM2 8-12 CsM2 12-17 Mälaren Mälaren Mälaren Mälaren Mälaren Mälaren Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Oxic Oxic Oxic Oxic Oxic Oxic 0-7 7-12 12-17 0-4 8-12 12-17 <20 <20 <20 <20 <20 <20 <26 <26 <26 <26 <26 <26 21 <12 <12 <12 <12 <12 4.6 <4.5 <4.5 <4.5 <4.5 5.5 <62 <62 <62 <62 <62 <62 MnT1 30-34 MnT1 25-30 MnT1 20-25 CsT1 0-2 CsT1 2-4 CsT1 4-8 CsT1 8-14 Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Core 2 Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic 0-4 4-9 9-14 0-2 2-4 4-8 8-14 <20 <20 <20 <20 <20 <20 <20 <26 <26 <26 <26 <26 <26 <26 21 16 <12 <12 <12 <12 <12 13 11 <4.5 <4.5 <4.5 <4.5 <4.5 <62 <62 <62 <62 <62 <62 <62 MnT2 20-25 CsT2 0-2 CsT2 2-4 CsT2 4-6 Torsbyfjärden Torsbyfjärden Torsbyfjärden Torsbyfjärden Core 1 Core 2 Core 2 Core 2 Oxic Oxic Oxic Oxic 0-5 0-2 2-4 4-6 <20 <20 <20 <20 <26 <26 <26 <26 <12 <12 <12 <12 <4.5 <4.5 4.9 <4.5 <62 <62 <62 <62 MnS1 25-30 MnS1 20-25 MnS1 10-15 CsS1 0-2 CsS1 6-8 CsS1 12-15 Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Saltsjön Core 1 Core 1 Core 1 Core 2 Core 2 Core 2 Anoxic Anoxic Anoxic Anoxic Anoxic Anoxic 0-5 5-10 10-15 0-2 6-8 12-15 56 21 56 32.2 22.2 <20 33 32 <26 <26 <26 <26 32 16 26 14 <12 <12 4.3 5.0 10 <4.5 5.2 8.4 126 74 92 <62 <62 <62 MnS2 24-26 CsS2 0-2 Saltsjön Saltsjön Core 1 Core 2 Oxic Oxic 0-2 0-2 <20 <20 <26 <26 <12 <12 <4.5 <4.5 <62 <62 *Smaller than (<) values refers to lowest detection limit (LOD= S/N). 45