Chapter 20 Study guide Name: 1. What is meant by entropy
advertisement

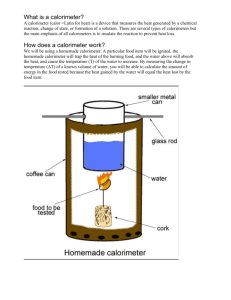
Chapter 20 Study guide Name:_____________________ 1. What is meant by entropy? Entropy refers to disordered energy given off during a chemical reaction. Because it is disordered, it cannot be harnessed to do work. 2. What does H stand for? H? H stands for enthalpy which refers to the total amount of energy present in a system. This is very difficult so scientists often times refer to the change in enthalpy, H, which refers to the energy released or absorbed during a chemical reaction. This value is easier to measure. 3. What is the difference between a positive H and a negative reaction involved? H in terms of energy and the kind of A positive H value means the reaction is endothermic and energy has been absorbed. A negative H value means the reaction is exothermic and energy has been released. 4. Why is energy needed to start exothermic reactions but not sustain them? All reactions require an input of energy to get them started (activation energy). Since exothermic reactions release energy, they are able to sustain themselves. The energy released provides activation energy to keep the process going. 5. Describe the general trends of spontaneous reactions in terms of energy and entropy changes. If a process is exoenergetic and entropy increases, it will occur spontaneously. (log burning) If a process is endoenergetic and entropy decreases, it will not occur spontaneously. (photosynthesis) 6. The specific heat of lead, c, is 0.128J/goC. How much heat is required to raise the temperature of 10g of lead form 20oC to 30oC? q=c X m X T = (0.128J/goC)(10g)(10oC) = 12.8J 7. What is specific heat? Specific heat, c, is the amount of heat, in joules, a substance requires to raise 1 gram of it by 1oC. 8. What is heat? How is heat measured? Heat is energy that flows from an area of high temperature to an area of low temperature, measured in joules. (or calories) 9. How does a calorimeter work? Calorimeters contain a measured mass of water and a thermometer. A substance of known mass is added to a calorimeter (heated) and the heat absorbed by the water is calculated. This can be used to determine the specific heat of a substance or the amount of calories present in a sample of food. 10. If a food contains 300 Calories per serving, how many calories is this? Joules? (1Calorie = 1000 calories, 4.184J = 1 calorie) 300 Calories X 1000 cal = 300,000 calories 1 1 Cal 300,000 calories X 4.184J = 1,255,200 Joules 1 1 cal 11. Describe the graph of an exothermic reaction. Heat is given off, reactants have more energy than products, so the graph is “down hill”. `12. Describe the graph of an endothermic reaction. Heat is absorbed, reactants have less energy than the products, so the graph is “up hill”. 13. Distinguish between the system and the surroundings. The system refers to the atoms and molecules undergoing the change or process. The surroundings include everything else. 14. Distinguish between the Calories listed on food labels and scientific calories. The Calories listed on food labels are kilocalories which are equal to 1000 scientific calories. 15. How are the Calories on food labels determined? They are determined by using a calorimeter.