Checklist – Research Subject to Department of Defense Regulations
advertisement
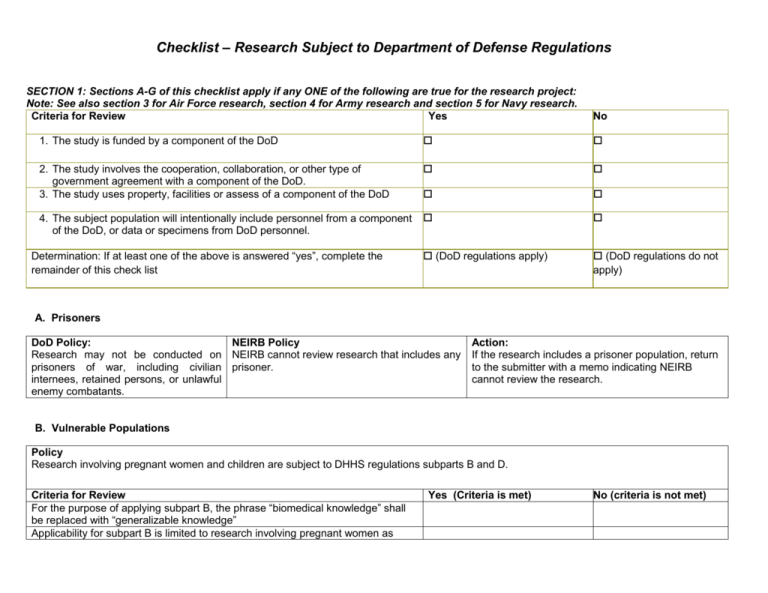
Checklist – Research Subject to Department of Defense Regulations SECTION 1: Sections A-G of this checklist apply if any ONE of the following are true for the research project: Note: See also section 3 for Air Force research, section 4 for Army research and section 5 for Navy research. Criteria for Review Yes No 1. The study is funded by a component of the DoD 2. The study involves the cooperation, collaboration, or other type of government agreement with a component of the DoD. 3. The study uses property, facilities or assess of a component of the DoD 4. The subject population will intentionally include personnel from a component of the DoD, or data or specimens from DoD personnel. (DoD regulations apply) (DoD regulations do not apply) Determination: If at least one of the above is answered “yes”, complete the remainder of this check list A. Prisoners DoD Policy: NEIRB Policy Research may not be conducted on NEIRB cannot review research that includes any prisoners of war, including civilian prisoner. internees, retained persons, or unlawful enemy combatants. Action: If the research includes a prisoner population, return to the submitter with a memo indicating NEIRB cannot review the research. B. Vulnerable Populations Policy Research involving pregnant women and children are subject to DHHS regulations subparts B and D. Criteria for Review For the purpose of applying subpart B, the phrase “biomedical knowledge” shall be replaced with “generalizable knowledge” Applicability for subpart B is limited to research involving pregnant women as Yes (Criteria is met) No (criteria is not met) subjects if the research is more than minimal risk and includes interventions or invasive procedures to the women or fetus; or if the research involves fetuses or neonates as subjects. Fetal research must comply with the US Code Title 42, Chapter 6A, subchapter III, part H, 289g (http://www.gpo.gov/fdsys/granule/USCODE-2010-title42/USCODE-2010-title42chap6A-subchapIII-partH-sec289g-2) Research involving children cannot be exempt. C. Waiver of Consent Policy Research involving a human being as an experimental subject, informed consent must be obtained in advance from the experimental subject or the subject’s legal representative consistent with part 219 of Reference (c) if the subject cannot consent. If consent is to be obtained from the experimental subject’s legal representative, the research must intend to benefit the individual subject. For the purpose of this policy, research involving a human being as an experimental subject is: “an activity for research purposes, where there is an intervention or interaction with a human being for the primary purpose of obtaining data regarding the effect of the intervention or interaction (32CFR.210.102 (f) reference (c). Examples of interventions or interactions include, but are not limited to: a physical procedure, a drug, a manipulation of the subject or subject’s environment, the withholding of an intervention that would have been undertaken if not for the research purpose.” Criteria for Review Yes (Criteria is met) No (criteria is not met) For research in which there is an intervention or interaction with a living individual for the primary purpose of obtaining data regarding the effect of the intervention or interaction, consent cannot be waived. For research in which there is an intervention or interaction with a living individual for the primary purpose of obtaining data regarding the effect of the intervention or interaction and consent will be obtained from subject’s legal representative, the research is intended to benefit the subject. If consent will be obtained from subject’s legal representative, provide protocol specific findings to support the determination that the research is intended to benefit the subject: Notes: Consent can be waived for screening of records for the purpose of identifying potential subjects, and does not apply to retrospective records review. Page 2 of 9 NEIRB # _____ For research involving a human being as an experimental subject, the Assistant Secretary of Defense for Research and Engineering component can waive consent under certain conditions. D. Payment to Active Duty Personnel Policy Yes (Criteria is met) Active duty personnel (i.e., not on leave or off-duty during participation) may not receive payment for participation except for blood donation. Payment for blood donation may not exceed $50 per blood draw. Note: Non-federal subjects can be compensated in accordance with NEIRB policy. No (criteria is not met) E. Classified Research Involving Human Subjects Policy: DOD-conducted non-exempt research involving human subjects that involves classified information must follow all of the requirements listed below. Executive order 13526 describes classified information (http://www.whitehouse.gov/the-press-office/executive-order-classifiednational-security-information). Requirement Involvement of classified information is limited to information needed for IRB approval and oversight of the research; information needed to inform human subjects during the consent process; and information provided to human subjects during the course of the research. After IRB approval of the research, Secretary of Defense approval is required. Waivers of consent are not allowed The informed consent process will included: Identification of the DoD as the supporting institution of the research, unless the research involves no more than minimal. (The Secretary of Defense may grant an exception in certain cases). Disclosure or use of classified information must comply with the requirements of Executive Order 13526 for access and protection of classified information. Yes (Requirement is met) No (criteria is not met) Expedited review is not allowed; Full Board review is always required for classified research. At least one non-affiliated member will be a non-federal employee. Page 3 of 9 NEIRB # _____ Any IRB member who disagrees with a majority decision approving the research may appeal the decision to the Secretary of Defense. The IRB will determine whether potential human subjects need access to classified information to make a valid, informed consent decision. F. Testing of Chemical or Biological Agents Policy Yes (Criteria is met) The research cannot involve the testing of chemical or biological agents. Note: Possible exceptions can be made as described in section 4.4.5 of the DoD Directive 3216.02. No (criteria is not met) G. Surveys or Questionnaires Administered to DOD Personnel or Their Families Policy Yes (Criteria is met) No (criteria is not met) Not Applicable DoD review and approval is required. IRB review may occur prior to DoD approval of the materials. Page 4 of 9 NEIRB # _____ SECTION 2 Is the study greater than minimal risk under DoD definition? Minimal Risk generally means that the probability and magnitude of physical or psychological harm anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life, or in routine medical, dental, or psychological examinations. However, for purposes of DoD research, “ordinarily encountered in daily life or during the performance of routine physical or physiological examinations or tests” shall not be interpreted to include the inherent risks certain categories of human subjects face in their everyday life. For example, the risks imposed in research involving human subjects focused on a special population should not be evaluated against the inherent risks encountered in their work environment (e.g., emergency responder, pilot, soldier in a combat zone) or having a medical condition (e.g., frequent medical tests or constant pain). No Yes: Complete sections H-J H. Research Monitor Policy: For more than minimal risk research, an independent monitor must be appointed by name for the research. The required monitor should be: A medical research monitor A non-medical research monitor: (provide justification for non-medical monitor): Yes (Criteria is met) No (criteria is not met) The monitor’s duties and responsibilities are described, including the following: - Subject recruitment and consent - Subject enrollment - Study procedures - Confidentiality - Adverse events and protocol deviations/violations - Data and specimen collection and storage - Data analysis The activities that must be performed by the monitor are described, including: - Discuss research project with the lead researcher Page 5 of 9 NEIRB # _____ - Interview some or all subjects Consult on individual cases Evaluate adverse event reports The monitor’s independence and authority are described, including whether the monitor: - is independent of the research team - possesses sufficient education and professional experience to serve as the subject advocate - will promptly report discrepancies or problems to the IRB - has the authority to stop the research process, remove individual subjects from the research, and take whatever steps are necessary to protect the safety and wellbeing of the subjects until the IRB can assess the report. The monitor’s full name is included in the research The monitor’s CV is included in the research A letter from the monitor accepting the role is included in the research I. Research Related Injury Policy The arrangement for emergency treatment and necessary follow-up of any research related injury is described. Yes (Criteria is met) No (criteria is not met) J. Research Involving Military Personnel Policy: All greater than minimal risk research involving military personnel must include the following additional protections Yes (Criteria is met) No (criteria is not met) Not Applicable (no military personnel will be enrolled) The researcher will ascertain than an individual’s decision about participation has not been influenced by unit officers or senior noncommissioned officers (NCOs). The research will exclude unit officers and senior NCOs from solicitation/recruitment/consent sessions for units under their command. The research will offer separate recruitment/consent sessions for officers and NCOs excluded from sessions held for their units. An ombudsperson not connected in any way to the research or to the unit will be present to monitor group recruitment briefings where a percentage of the unit is Page 6 of 9 NEIRB # _____ being recruited to participate as a group, to monitor that the voluntary nature of individual participates in adequately stressed and that the information provided about the research is adequate and true. Section 3: Air Force Research Complete this section for Air Force research only. Policy: Research involving the Air Force must comply with these limitations: The researcher must consult with each subject to determine whether participation in the research would affect the subject’s ability to mobilize for readiness, to perform duties, or to be available for duty. (Normally, if the participation could affect these abilities, the subject should not be considered for participation in the research.) Apparatus, instruments and personnel will be available to deal with medical emergencies related to the research. Minors may be used as subjects only when the research is intended to be of benefit to the subjects and satisfies one of the following: Involves no more than minimal risk to subjects; OR Yes (Criteria is met) No (criteria is not met) Not Applicable Presents greater than minimal risk but presents the prospect of direct benefit for individuals subjects and the IRB finds that: - the risk is justified by the anticipated benefit to subject. - the relationship of the anticipated benefit to the risk is at least favorable to the subjects as that presented by available alternative approaches. A mentally disabled or institutionalized mentally infirm person may not participate as a subject, unless the study would be impossible or meaningless if such subjects were excluded. A mentally disabled or institutionalized mentally infirm person must give legally effective consent, or their legal guardian must give effective consent, according to local law. A mentally disabled or institutionalized mentally infirm person can only be enrolled if the research is concerned with one or more of the following: - the diagnosis, treatment, prevention or etiology of a particular impairment that inflicts the subjects. Page 7 of 9 NEIRB # _____ - Any other condition, from which subjects are suffereing, provided there is a direct benefit to the subjects and prior testing has proved the risk to be acceptable. - The effect of institutional life on the institutionalized mentally infirm subjects and involves no appreciable risk to subjects. Surveys which collect data through interaction with subjects or surveys which contain identifiable private information are not exempt from IRB review. For Air Force research, the IRB must specify the research focus area(s) of the study; select all options below that apply: Medical Readiness Prevention Medium Utilization managed Care Treatment, Diagnosis or Other Section 4: Army Research Complete this section for Army research only. Policy: Research involving the Army must comply with these limitations: Yes (Criteria is met) No (criteria is not met) Not Applicable Minors may be used as subjects only when the research is intended to be of benefit to the subjects and satisfies one of the following: Involves no more than minimal risk to subjects; OR Presents greater than minimal risk but presents the prospect of direct benefit for individuals subjects and the IRB finds that: - the risk is justified by the anticipated benefit to subject. - the relationship of the anticipated benefit to the risk is at least favorable to the subjects as that presented by available alternative approaches. Drugs, placebos, biologicals and vaccines not commercially available (i.e., investigational) will be received, stored and controlled by the pharmacy and will not be dispensed without an approved protocol. There is documentation of independent review and approval for scientific merit or scholarship (including a summary of scientific issues raised and addressed during the review). The entity that conducted the independent review must be specified and may include agencies such as: NIH, NSF, other funding agency or a faculty Page 8 of 9 NEIRB # _____ sponsor. Section 5: Navy Research Complete this section for Navy research only. Policy: Research involving the Navy must comply with these limitations: Yes (Criteria is met) No (criteria is not met) Not Applicable Regardless of the level of research, no superiors (supervisors, officers, NCOs), will influence the decisions of their subordinates (e.g., junior enlisted personnel) whether to participate as study subjects. When conducting multi-site research, the Navy requires a formal agreement between the organizations to specify the research, specific roles and responsibilities of the institution responsibility for scientific and IRB review, recruitment for subjects, and procedures for obtaining consent. There is documentation of independent review and approval for scientific merit or scholarship (including a summary of scientific issues raised and addressed during the review). The entity that conducted the independent review must be specified and may include agencies such as: NIH, NSF, other funding agency or a faculty sponsor. Page 9 of 9 NEIRB # _____







