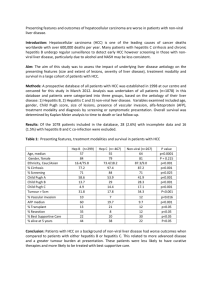
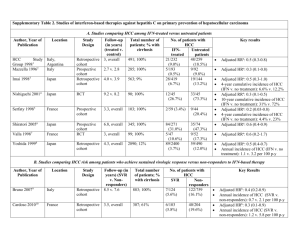
The main risk factors for hepatocellular carcinoma are
advertisement

Hepatocellular carcinoma Hepatocellular carcinoma (HCC, also called malignant hepatoma) is the most common type of liver cancer. Most cases of HCC are secondary to either a viral hepatitis infection (hepatitis B or C) or cirrhosis (alcoholism being the most common cause of hepatic cirrhosis). Compared to other cancers, HCC is quite a rare tumour in the United States. In countries where hepatitis is not endemic, most malignant cancers in the liver are not primary HCC but metastasis (spread) of cancer from elsewhere in the body, e.g., the colon. Treatment options of HCC and prognosis are dependent on many factors but especially on tumour size and staging. Tumour grade is also important. High-grade tumours will have a poor prognosis, while lowgrade tumors may go unnoticed for many years, as is the case in many other organs. Signs and symptoms HCC may present with jaundice, bloating from ascites, easy bruising from blood clotting abnormalities or as loss of appetite, unintentional weight loss, abdominal pain, especially in the right upper quadrant, nausea, emesis, or fatigue. Risk factors The main risk factors for hepatocellular carcinoma are; Alcoholism Hepatitis B Hepatitis C (25% of causes globally) Aflatoxin Cirrhosis of the liver Hemochromatosis Wilson's disease (while some theorise the risk increases, case studies are rare and suggest the opposite where Wilson's disease actually may confer protection) Type 2 Diabetes (probably aided by obesity) The risk factors which are most important varies widely from country to country. In countries where Hepatitis B is endemic, such as China, Hepatitis B will be the predominant cause of Hepatocellular Carcinoma. Whereas in countries, such as the United States, where Hepatitis B is rare because of high vaccination rates, the major cause of HCC is Cirrhosis (often due to alcohol abuse). The risk of hepatocellular carcinoma in type 2 diabetics is greater (from 2.5 to 7.1 times the non diabetic risk) depending on the duration of diabetes and treatment protocol. A suspected contributor to this increased risk is circulating insulin concentration such that diabetics with poor insulin control or on treatments that elevate their insulin output (both states that contribute to a higher circulating insulin concentration) show far greater risk of hepatocellular carcinoma than diabetics on treatments that reduce circulating insulin concentration. On this note, some diabetics who engage in tight insulin control (by keeping it from being elevated) show risk levels low enough to be indistinguishable from the general population. This phenomenon is thus not isolated to diabetes mellitus type 2 since poor insulin regulation is also found in other conditions such as metabolic syndrome (specifically, when evidence of non alcoholic fatty liver disease or NAFLD is present) and again there is evidence of greater risk here too. While there are claims that anabolic steroid abusers are at greater risk (theorized to be due to insulin and IGF exacerbation), the only evidence that has been confirmed is that anabolic steroid users are more likely to have hepatocellular adenomas (a benign form of HCC) transform into the more dangerous hepatocellular carcinoma. When hepatocellular adenomas grow to a size of more than 6–8 cm, they are considered cancerous and thus become a risk of hepatocellular carcinoma. Although hepatocellular carcinoma most commonly affects adults, children who are affected with biliary atresia, infantile cholestasis, glycogen-storage diseases, and other cirrhotic diseases of the liver are predisposed to developing hepatocellular carcinoma. Children and adolescents are unlikely to have chronic liver disease, however, if they suffer from congenital liver disorders, this fact increases the chance of developing hepatocellular carcinoma. Young adults afflicted by the rare fibrolamellar variant of hepatocellular carcinoma may have none of the typical risk factors, i.e. cirrhosis and hepatitis. Pathogenesis Hepatocellular carcinoma, like any other cancer, develops when there is a mutation to the cellular machinery that causes the cell to replicate at a higher rate and/or results in the cell avoiding apoptosis. In particular, chronic infections of hepatitis B and/or C can aid the development of hepatocellular carcinoma by repeatedly causing the body's own immune system to attack the liver cells, some of which are infected by the virus, others merely bystanders. While this constant cycle of damage followed by repair can lead to mistakes during repair which in turn lead to carcinogenesis, this hypothesis is more applicable, at present, to hepatitis C. Chronic hepatitis C causes HCC through the stage of cirrhosis. In chronic hepatitis B, however, the integration of the viral genome into infected cells can directly induce a non-cirrhotic liver to develop HCC. Alternatively, repeated consumption of large amounts of ethanol can have a similar effect. Besides, cirrhosis is commonly caused by alcoholism, chronic hepatitis B and chronic hepatitis C. The toxin aflatoxin from certain Aspergillus species of fungus is a carcinogen and aids carcinogenesis of hepatocellular cancer by building up in the liver. The combined high prevalence of rates of aflatoxin and hepatitis B in settings like China and West Africa has led to relatively high rates of heptatocellular carcinoma in these regions. Other viral hepatitides such as hepatitis A have no potential to become a chronic infection and thus are not related to hepatocellular carcinoma. Diagnosis Hepatocellular carcinoma (HCC) most commonly appears in a patient with chronic viral hepatitis (hepatitis B or hepatitis C, 20%) or/and with cirrhosis (about 80%). These patients commonly undergo surveillance with ultrasound due to the cost-effectiveness. In patients with a higher suspicion of HCC (such as rising alpha-fetoprotein and des-gamma carboxyprothrombin levels), the best method of diagnosis involves a CT scan of the abdomen using intravenous contrast agent and three-phase scanning (before contrast administration, immediately after contrast administration, and again after a delay) to increase the ability of the radiologist to detect small or subtle tumors. It is important to optimize the parameters of the CT examination, because the underlying liver disease that most HCC patients have can make the findings more difficult to appreciate. On CT, HCC can have three distinct patterns of growth: A single large tumor Multiple tumors Poorly defined tumor with an infiltrative growth pattern A biopsy is not needed to confirm the diagnosis of HCC if certain imaging criteria are met. The key characteristics on CT are hypervascularity in the arterial phase scans, washout or de-enhancement in the portal and delayed phase studies, a pseudocapsule and a mosaic pattern. Both calcifications and intralesional fat may be appreciated. CT scans use contrast agents, which are typically iodine or barium based. Some patients are allergic to one or both of these contrast agents, most often iodine. Usually the allergic reaction is manageable and not life threatening. An alternative to a CT imaging study would be the MRI. MRI's are more expensive and not as available because fewer facilities have MRI machines. More important MRI are just beginning to be used in tumor detection and fewer radiologists are skilled at finding tumors with MRI studies when it is used as a screening device.[citation needed] Mostly the radiologists are using MRIs to do a secondary study to look at an area where a tumor has already been detected.[citation needed] MRI's also use contrast agents. One of the best for showing details of liver tumors is very new: iron oxide nano-particles appears to give better results.[citation needed] The latter are absorbed by normal liver tissue, but not tumors or scar tissue.[citation needed] In a review article of the screening, diagnosis and treatment of hepatocellular carcinoma, 4 articles were selected for comparing the accuracy of CT and MRI in diagnosing this malignancy. Radiographic diagnosis was verified against post-transplantation biopsy as the gold standard. With the exception of one instance of specificity, it was discovered that MRI was more sensitive and specific than CT in all four studies. Pathology Macroscopically, liver cancer appears as a nodular or infiltrative tumor. The nodular type may be solitary (large mass) or multiple (when developed as a complication of cirrhosis). Tumor nodules are round to oval, grey or green (if the tumor produces bile), well circumscribed but not encapsulated. The diffuse type is poorly circumscribed and infiltrates the portal veins, or the hepatic veins (rarely). Microscopically, there are four architectural and cytological types (patterns) of hepatocellular carcinoma: fibrolamellar, pseudoglandular (adenoid), pleomorphic (giant cell) and clear cell. In well differentiated forms, tumor cells resemble hepatocytes, form trabeculae, cords and nests, and may contain bile pigment in cytoplasm. In poorly differentiated forms, malignant epithelial cells are discohesive, pleomorphic, anaplastic, giant. The tumor has a scant stroma and central necrosis because of the poor vascularization. Staging Important features that guide treatment include: size spread (stage) involvement of liver vessels presence of a tumor capsule presence of extrahepatic metastases presence of daughter nodules vascularity of the tumor MRI is the best imaging method to detect the presence of a tumor capsule. Prevention Since hepatitis B or C is one of the main causes of hepatocellular carcinoma, prevention of this infection is key to then prevent hepatocellular carcinoma. Thus, childhood vaccination against hepatitis B may reduce the risk of liver cancer in the future. In the case of patients with cirrhosis, alcohol consumption is to be avoided. Also, screening for hemochromatosis may be beneficial for some patients. Management Liver transplantation to replace the diseased liver with a cadaveric liver or a living donor graft has historically low survival rates (20%-36%). During 1996–2001 the rate had improved to 61.1%, likely related to adoption of the Milan criteria at US transplantation centers. Expanded Shanghai criteria in China resulted in overall survival and disease-free survival rates similar to the Milan criteria. Studies from the late 2000 obtained higher survival rates ranging from 67% to 91%. If the liver tumor has metastasized, the immunosuppressant post-transplant drugs decrease the chance of survival. Considering this objective risk in conjunction with the potentially high rate of survival, some recent studies conclude that: "LTx can be a curative approach for patients with advanced HCC without extrahepatic metastasis". For those reasons, and others, it is considered nowadays that patient selection is a major key for success. A receptor tyrosine kinase inhibitor, Sorafenib, approved by the US FDA in December 2005 and in Europe in July 2006, may be used in patients with advanced hepatocellular carcinoma. Sorafenib is a small molecule that inhibits tumor-cell proliferation and tumor angionesis. It has been shown in a Spanish phase III clinical trial to add two months to the lifespan of late stage HCC patients with well preserved liver function. It also increases the rate of apoptosis in other tumor models. The results indicated that singleagent sorafenib might have a beneficial therapeutic effect. In this study, for instance, the median overall survival was of 9.2 months and the median time to progression was of 5.5 months. Also, the survival benefit represented a 31% relative reduction in the risk of death. Surgical resection to remove a tumor together with surrounding liver tissue while preserving enough liver remnant for normal body function. This treatment offers the best prognosis for long-term survival, but only 10-15% of patients are suitable for surgical resection. This is often because of extensive disease or poor liver function. Resection in cirrhotic patients carries high morbidity and mortality. The expected liver remnant should be more than 25% of the total size for a non-cirrhotic liver, while that should be more than 40% of the total size for a cirrhotic liver. The overall recurrence rate after resection is 50-60%. Percutaneous ethanol injection (PEI) well tolerated, high RR in small (<3 cm) solitary tumors; as of 2005, no randomized trial comparing resection to percutaneous treatments; recurrence rates similar to those for postresection. However a comparative study found that local therapy can achieve a 5-year survival rate of around 60% for patients with small HCC. Transcatheter arterial chemoembolization (TACE) is usually performed for unresectable tumors or as a temporary treatment while waiting for liver transplant. TACE is done by injecting an antineoplastic drug (e.g. cisplatin) mixed with a radioopaque contrast (e.g. Lipiodol) and an embolic agent (e.g. Gelfoam) into the right or left hepatic artery via the groin artery. As of 2005, multiple trials show objective tumor responses and slowed tumor progression but questionable survival benefit compared to supportive care; greatest benefit seen in patients with preserved liver function, absence of vascular invasion, and smallest tumors. TACE is not suitable for big tumors (>8 cm), presence of portal vein thrombus, tumors with portal-systemic shunt and patients with poor liver function. Radiofrequency ablation (RFA) uses high frequency radio-waves to destroy tumor by local heating. The electrodes are inserted into the liver tumor under ultrasound image guidance using percutaneous, laparoscopic or open surgical approach. It is suitable for small tumors (<5 cm). A large randomised trial comparing surgical resection and RFA for small HCC showed similar 4 years-survival and less morbidities for patients treated with RFA. Focused External Beam Radiation Stereotactic Radiotherapy (SRT) is a technique of using highly focussed radiation to small target volume. SRT has been tried successfully in the liver for treatment of metastases, and currently clinical studies are underway to evaluate its efficacy in treating Hepatocellular Carcinoma. The early results are promising. With the advent of modern computer technology, it is now possible to direct treatment to involved areas of the liver, while sparing normal healthy liver tissue. Selective internal radiation therapy can be used to destroy the tumor from within (thus minimizing exposure to healthy tissue). There are currently two products available, SIR-Spheres and TheraSphere The latter is an FDA approved treatment for primary liver cancer (HCC) which has been shown in clinical trials to increase survival rate of low-risk patients. SIR-Spheres are FDA approved for the treatment of metastatic colorectal cancer but outside the US SIR-Spheres are approved for the treatment of any non-resectable liver cancer including primary liver cancer. This method uses a catheter (inserted by a radiologist) to deposit radioactive particles to the area of interest. Intra-arterial iodine-131–lipiodol administration Efficacy demonstrated in unresectable patients, those with portal vein thrombus. This treatment is also used as adjuvant therapy in resected patients (Lau at et, 1999). It is believed to raise the 3-year survival rate from 46 to 86%. This adjuvant therapy is in phase III clinical trials in Singapore and is available as a standard medical treatment to qualified patients in Hong Kong. Combined PEI and TACE can be used for tumors larger than 4 cm in diameter, although some Italian groups have had success with larger tumours using TACE alone. High intensity focused ultrasound (HIFU) (not to be confused with normal diagnostic ultrasound) is a new technique which uses much more powerful ultrasound to treat the tumour. Still at a very experimental stage. Most of the work has been done in China. Some early work is being done in Oxford and London in the UK. Hormonal therapy Antiestrogen therapy with tamoxifen studied in several trials, mixed results across studies, but generally considered ineffective Octreotide (somatostatin analogue) showed 13-month MS v 4-month MS in untreated patients in a small randomized study; results not reproduced. Adjuvant chemotherapy: No randomized trials showing benefit of neoadjuvant or adjuvant systemic therapy in HCC; single trial showed decrease in new tumors in patients receiving oral synthetic retinoid for 12 months after resection/ablation; results not reproduced. Clinical trials have varying results. Palliative: Regimens that included doxorubicin, cisplatin, fluorouracil, interferon, epirubicin, or taxol, as single agents or in combination, have not shown any survival benefit (RR, 0%-25%); a few isolated major responses allowed patients to undergo partial hepatectomy; no published results from any randomized trial of systemic chemotherapy. Cryosurgery: Cryosurgery is a new technique that can destroy tumors in a variety of sites (brain, breast, kidney, prostate, liver). Cryosurgery is the destruction of abnormal tissue using sub-zero temperatures. The tumor is not removed and the destroyed cancer is left to be reabsorbed by the body. Initial results in properly selected patients with unresectable liver tumors are equivalent to those of resection. Cryosurgery involves the placement of a stainless steel probe into the center of the tumor. Liquid nitrogen is circulated through the end of this device. The tumor and a half inch margin of normal liver are frozen to -190°C for 15 minutes, which is lethal to all tissues. The area is thawed for 10 minutes and then re-frozen to -190°C for another 15 minutes. After the tumor has thawed, the probe is removed, bleeding is controlled, and the procedure is complete. The patient will spend the first post-operative night in the intensive care unit and typically is discharged in 3 – 5 days. Proper selection of patients and attention to detail in performing the cryosurgical procedure are mandatory in order to achieve good results and outcomes. Frequently, cryosurgery is used in conjunction with liver resection as some of the tumors are removed while others are treated with cryosurgery. Patients may also have insertion of a hepatic intra-arterial catheter for post-operative chemotherapy. As with liver resection, the surgeon should have experience with cryosurgical techniques in order to provide the best treatment possible. Interventional radiology Agaricus blazei mushrooms inhibited abnormal collagen fiber formation in human hepatocarcinoma cells in an in vitro experiment. A systematic review assessed 12 articles involving a total of 318 patients with hepatocellular carcinoma treated with Yttrium-90 radioembolization. Excluding a study of only one patient, post-treatment CT evaluation of the tumor showed a response ranging from 29 to 100% of patients evaluated, with all but two studies showing a response of 71% or greater. Gallium maltolate demonstrated in vitro efficacy against several HCC cell lines[36] and produced very encouraging results in a clinical case of advanced HCC that had not responded to therapy with sorafenib. Gallium is known, from gallium scanning results, to be taken up preferentially by many hepatocellular carcinoma tumors. Therapeutic doses of gallium follow the same uptake pathway, causing inhibition of tumor growth and eventual apoptosis of tumor cells, while sparing healthy tissue. Prognosis The usual outcome is poor, because only 10–20% of hepatocellular carcinomas can be removed completely using surgery. If the cancer cannot be completely removed, the disease is usually deadly within 3 to 6 months. This is partially due to late presentation with large tumours, but also the lack of medical expertise and facilities. However, survival can vary, and occasionally people will survive much longer than 6 months. The prognosis for metastatic or unresectable hepatocellular carcinoma has recently improved due to the approval of sorafenib (Nexavar®) for advanced hepatocellular carcinoma. Epidemiology HCC is one of the most common tumors worldwide. The epidemiology of HCC exhibits two main patterns, one in North America and Western Europe and another in non-Western countries, such as those in sub-Saharan Africa, central and Southeast Asia, and the Amazon basin. Males are affected more than females usually and it is most common between the age of 30 to 50, Hepatocellular carcinoma causes 662,000 deaths worldwide per year about half of them in China. Africa and Asia In some parts of the world, such as sub-Saharan Africa and Southeast Asia, HCC is the most common cancer, generally affecting men more than women, and with an age of onset between late teens and 30s. This variability is in part due to the different patterns of hepatitis B and hepatitis C transmission in different populations - infection at or around birth predispose to earlier cancers than if people are infected later. The time between hepatitis B infection and development into HCC can be years, even decades, but from diagnosis of HCC to death the average survival period is only 5.9 months according to one Chinese study during the 1970-80s, or 3 months (median survival time) in Sub-Saharan Africa according to Manson's textbook of tropical diseases. HCC is one of the deadliest cancers in China where chronic hepatitis B is found in 90% of cases. In Japan, chronic hepatitis C is associated with 90% of HCC cases. Food infected with Aspergillus flavus (especially peanuts and corns stored during prolonged wet seasons) which produces aflatoxin poses another risk factor for HCC. North America and Western Europe Most malignant tumors of the liver discovered in Western patients are metastases (spread) from tumors elsewhere. In the West, HCC is generally seen as a rare cancer, normally of those with pre-existing liver disease. It is often detected by ultrasound screening, and so can be discovered by health-care facilities much earlier than in developing regions such as SubSaharan Africa. Acute and chronic hepatic porphyrias (acute intermittent porphyria, porphyria cutanea tarda, hereditary coproporphyria, variegate porphyria) and tyrosinemia type I are risk factors for hepatocellular carcinoma. The diagnosis of an acute hepatic porphyria (AIP, HCP, VP) should be sought in patients with hepatocellular carcinoma without typical risk factors of hepatitis B or C, alcoholic liver cirrhosis or hemochromatosis. Both active and latent genetic carriers of acute hepatic porphyrias are at risk for this cancer, although latent genetic carriers have developed the cancer at a later age than those with classic symptoms. Patients with acute hepatic porphyrias should be monitored for hepatocellular carcinoma. HCC is one of the most common tumors worldwide. The epidemiology of HCC exhibits two main patterns, one in North America and Western Europe and another in non-Western countries, such as those in sub-Saharan Africa, central and Southeast Asia, and the Amazon basin. Males are affected more than females usually and it is most common between the age of 30 to 50, Hepatocellular carcinoma causes 662,000 deaths worldwide per year about half of them in China. Africa and Asia In some parts of the world, such as sub-Saharan Africa and Southeast Asia, HCC is the most common cancer, generally affecting men more than women, and with an age of onset between late teens and 30s. This variability is in part due to the different patterns of hepatitis B and hepatitis C transmission in different populations - infection at or around birth predispose to earlier cancers than if people are infected later. The time between hepatitis B infection and development into HCC can be years, even decades, but from diagnosis of HCC to death the average survival period is only 5.9 months according to one Chinese study during the 1970-80s, or 3 months (median survival time) in Sub-Saharan Africa according to Manson's textbook of tropical diseases. HCC is one of the deadliest cancers in China where chronic hepatitis B is found in 90% of cases. In Japan, chronic hepatitis C is associated with 90% of HCC cases. Food infected with Aspergillus flavus (especially peanuts and corns stored during prolonged wet seasons) which produces aflatoxin poses another risk factor for HCC. North America and Western Europe Most malignant tumors of the liver discovered in Western patients are metastases (spread) from tumors elsewhere. In the West, HCC is generally seen as a rare cancer, normally of those with pre-existing liver disease. It is often detected by ultrasound screening, and so can be discovered by health-care facilities much earlier than in developing regions such as SubSaharan Africa. Acute and chronic hepatic porphyrias (acute intermittent porphyria, porphyria cutanea tarda, hereditary coproporphyria, variegate porphyria) and tyrosinemia type I are risk factors for hepatocellular carcinoma. The diagnosis of an acute hepatic porphyria (AIP, HCP, VP) should be sought in patients with hepatocellular carcinoma without typical risk factors of hepatitis B or C, alcoholic liver cirrhosis or hemochromatosis. Both active and latent genetic carriers of acute hepatic porphyrias are at risk for this cancer, although latent genetic carriers have developed the cancer at a later age than those with classic symptoms. Patients with acute hepatic porphyrias should be monitored for hepatocellular carcinoma. Pre-clinical Current research includes the search for the genes that are disregulated in HCC, protein markers, non-coding RNAs (such as TUC338) and other predictive biomarkers. As similar research is yielding results in various other malignant diseases, it is hoped that identifying the aberrant genes and the resultant proteins could lead to the identification of pharmacological interventions for HCC. Clinical JX-594, an oncolytic virus, has orphan drug designation for this condition and is undergoing clinical trials. HCC treatments in Phase II & Phase III Development at June 2013 Company Name 4SC AG Product Names 4SC-201 (Compound #), BYK408740 Descr iption Partners Latest Stage of Develo pment Oral pan- Yakult histone Honsha Co. Phase II deacetylas Ltd. e (HDAC) Indication Details First-line treatment of advanced hepatocellular carcinoma (HCC); Company Name Product Names Descr iption Partners Latest Stage of Develo pment (Former inhibitor compound #),resminostat (Generic) Active Biotech AB Second-line treatment of hepatocellular carcinoma (HCC) Oral quinoline-3carboxamid ABR-215050 e derivative (Compound that binds #),tasquinimo S100 Ipsen Group Phase II d (Generic), calcium TASQ binding protein A9 (Informal) (S100A9; calgranulin B; MRP14) SGI-110 Astex (Compound Hypomethy Pharmaceutic #), S110 lating agent (Former als Inc. compound #) AstraZeneca plc BAY 86-9766 (Compound #), RDEA119 (Former compound #),Refametinib (Informal) Eli Lilly and Co. LY2157299 Transformin (Compound #) g growth Selective inhibitor of mitogenactivated ERK kinase (MEK) Indication Details Bayer AG Treat advanced or metastatic hepatocellular carcinoma (HCC) Phase II Treat advanced hepatocellular carcinoma (HCC) Phase II Treat hepatocellular carcinoma (HCC) Phase II Treat hepatocellular carcinoma (HCC) Company Name Product Names Descr iption Partners Latest Stage of Develo pment Indication Details factor (TGF) beta receptor 1 (TGFBR1; ALK5) inhibitor GenSpera Inc. Prodrug of plantG-202 derived (Compound #) cytotoxin 12ADT Tykerb (Brand), Tyverb (Brand), GW572016 GlaxoSmithKlin (Compound #), lapatinib(Ge e plc neric), Tykerb (Other), Tyverb (Other) HER1 and HER2 receptor kinase inhibitor Phase II Treat advanced, progressive hepatocellular carcinoma (HCC) Eddingpharm Phase II Inc. Treat hepatocellular carcinoma (HCC) Phase II Treat hepatocellular carcinoma (HCC) Novartis AG Phase II Treat advanced hepatocellular carcinoma (HCC) JX-594 Recombinan Transgene Jennerex (Compound #), t vaccinia S.A.;Green Biotherapeutic TG6006 virus Cross Corp.; Phase II Treat advanced hepatocellular cancer (HCC); Treat Green Cross Corp. Engineered JX594 oncolytic (Compound #) virus Incyte Corp. INC280 (Compound #), INCB28060 (Former compound #) Oral c-Met receptor tyrosine kinase inhibitor Company Name s Inc. Product Names (Compound #),pexastimoge ne devacirepvec ( Generic), PexaVec (Informal), JX-5940TG6006 (Other) Descr iption (addition of GM-CSF and deletion of thymidine kinase) Recombinan t fusion protein that NGR-hTNF selectively (Compound binds to MolMed S.p.A. #), Arenegyr (F alanyl membrane ormer) aminopeptid ase (ANPEP; APN; CD13) Partners Latest Stage of Develo pment Lee's Pharmaceutic al Holdings Ltd. Indication Details hepatocellular carcinoma (HCC); Treat primary liver cancer or cancer metastatic to the liver; Treat unresectable primary hepatocellular carcinoma (HCC) Phase II Treat hepatocellular carcinoma (HCC) Phase II Treat metastatic hepatocellular carcinoma (HCC) Novartis AG SOM230 (Compound #),pasireotide ( Somatostati Generic), n analog Signifor (Informal) Pfizer Inc. CP-675 (Compound #), Human mAB CP-675,206 AstraZeneca Phase II (Compound #), against plc CTLA-4 CP-675206 (Compound #), ticilimumab Treat hepatocellular carcinoma (HCC) Company Name Product Names Descr iption Partners Latest Stage of Develo pment Indication Details (Former),treme limumab (Infor mal) Lansoprazol e lansoprazole (G formulated Innovus Apricus eneric), with Pharmaceutical Biosciences PrevOnco NexMed's s Inc. Inc. NexACT (Informal) delivery technology AbbVie Inc. Inhibitor of vascular endothelial growth ABT-869 factor (Compound (VEGF) and #),linifanib (Ge platelet derived neric) growth factor (PDGF) receptor ArQule Inc. Small molecule ARQ 197 inhibitor of (Compound c-Met #), tivantinib (G receptor eneric) tyrosine kinase Daiichi Sankyo Co. Ltd.; Kyowa Hakko Kirin Co. Ltd. Phase II/III Treat hepatocellular carcinoma (HCC) Phase III Treat advanced or metastatic hepatocellular carcinoma (HCC); Treat liver cancer Phase III Treat hepatocellular carcinoma (HCC); Treat unresectable hepatocellular carcinoma (HCC) in patients who have failed one prior systemic therapy Company Name Product Names Descr iption Astellas Pharma Inc. Tarceva (Brand), R1415 (Compound #), RG115 (Compound #), CP-358,774 (Former compound #), OSI-774 (Former compound #),erlotinib (Ge neric), Tarceva (Other) Small molecule inhibitor of EGFR tyrosine kinase activity Bayer AG Stivarga (Brand), BAY 73-4506 (Compound #),regorafenib ( Generic), DAST Inhibitor (Informal), fluorosorafenib (Other) Dual acting signal transduction (DAST) inhibitor of multiple kinases BioAlliance Pharma S.A. BA-003 (Compound #),doxorubicin (Generic), Livatag doxorubicin Transdrug Nanoparticle formulation of doxorubicin Partners Latest Stage of Develo pment Chugai Pharmaceutic al Co. Phase III Ltd;Genentec h Inc.;Roche Indication Details Treat hepatocellular carcinoma (HCC) Phase III Treat advanced hepatocellular carcinoma (HCC); Treat hepatocellular carcinoma (HCC) Phase III Treat advanced hepatocellular carcinoma (HCC); Treat hepatocellular carcinoma (HCC) Company Name Product Names Descr iption Partners Latest Stage of Develo pment Indication Details (Other) Bristol-Myers Squibb Co. Dual inhibitor of VEGFR-2 BMS-582664 and (Compound fibroblast #), Brivanib(Ot growth factor (FGF) her) receptor 1 (FGFR1; CD331) Phase III Celsion Corp. Doxorubicin ThermoDox encapsulate heat-activated d in a heatliposome activated (Informal) liposome Yakult Honsha Co. Ltd.; Zhejiang Phase III Hisun Pharmaceutic al Co. Ltd. Phase III Treat hepatocellular carcinoma (HCC) SFJ Pharmaceutic Phase III als Inc. Treat hepatocellular carcinoma (HCC) Delcath Systems Inc. Chemostat doxorubicin (Informal) Doxorubicin delivered using the Chemosat percutaneou s hepatic perfusion system Eisai Co. Ltd. E7080 (Compound #),Lenvatinib ( Other) Inhibitor of multiple VEGF receptor tyrosine First- and second-line treatment of hepatocellular cancer (HCC); First-line treatment of hepatocellular carcinoma (HCC); Treat hepatocellular carcinoma (HCC) Treat colorectal liver metastases; Treat liver cancer; Treat metastatic liver cancer; Treat nonresectable hepatocellular carcinoma (HCC) Company Name Product Names Descr iption Partners Latest Stage of Develo pment Indication Details kinases IMC-1121B (Compound #), Human IgG1 LY3009806 mAb VEGFREli Lilly and Co. (Compound 2 antagonist #),ramuciruma b (Generic) Phase III Treat advanced, inoperable liver cancer in treatment-naïve patients; Treat hepatocellular carcinoma (HCC) Phase III Prevent recurrence after curative treatment of HCV-related hepatocellular carcinoma (HCC); Prevent recurrence of hepatocellular carcinoma (HCC) in patients with HCV; Treat hepatocellular cancer (HCC) Phase III Treat hepatoma; Treat liver metastases from colorectal cancer; Treat unresectable hepatocellular carcinoma (HCC) Sulfated mannopenta Medigen Biotechnolog Phase III ose phosphate y Corp. anti- Adjuvant treatment of hepatitis virus-related hepatocellular carcinoma (HCC) after surgical resection; Treat Kowa Co. Ltd. K-333 (Compound #), NIK-333 (Compound #),peretinoin ( Generic), Ruchiko (Other) Light Sciences Oncology Inc. Photodynam ic therapy Litx (PDT) using (Former), Apto photosensiti cine zing agent talaporfin sodi talaporfin um (Informal) sodium (LS11) PI-88 Progen (Compound Pharmaceutical #),muparfostat s Ltd. (Generic) Oral acyclic retinoid with a vitamin Alike structure Company Name Product Names Descr iption Partners Latest Stage of Develo pment angiogenic agent that inhibits VEGF, FGF and heparanase activity Teysuno (Brand ), S-1 (Compound #), TS-1 Taiho (Compound #), Pharmaceutical tegafur/gimera cil/oteracil Co. Ltd. potassium (Generic), Teysuno (Informal) hepatocellular carcinoma (HCC) following primary tumor resection; Treat primary liver cancer Oral combination of 5fluorouracil Nordic Group Phase III (5-FU) plus two enzyme inhibitors Lowmolecularweight antiTSU-68 Taiho angiogenetic (Compound Pharmaceutical agent that #),orantinib (G inhibits Co. Ltd. eneric) receptor tyrosine kinase Indication Details Phase III Treat hepatocellular carcinoma (HCC); Treat liver cancer Treat hepatocellular carcinoma (HCC) Abbreviations HCC, hepatocellular carcinoma; TACE, transarterial embolization/chemoembolization; PFS, progression-free survival; PS, performance status; HBV, hepatitis B virus; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; RR, response rate; MS, median survival.