Important Concepts to Remember Block 1 Unit 1 Introduction to Cell
advertisement

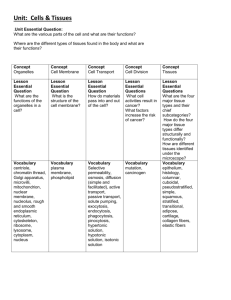
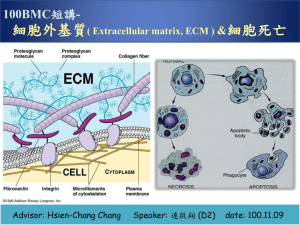
Important Concepts to Remember Block 1 Unit 1 Introduction to Cell Biology Maximum Resolving Power=0.22 µm Human cells ~ 10 µm Living cells can’t be seen under EM (black and white images) Organelle Smooth ER Size Rough ER Golgi Peroxisomes 0.2-1 µm Mitochondria Up to 7 µm Lysosome 0.3-0.8 µm Glycogen 10-40 nm Function Synthesis of steroids and detoxification of drugs; Ca++ accumulation and release Protein synthesis Accepts proteins from RER for modification Catabolizes long fatty acid chains Produces ATP; contains mitochondrial DNA and enzymes Contains acid hydrolases (has an acidic lumen) Storage form of glucose Six abnormal functions in the cell: 1. 2. 3. 4. 5. 6. Swollen mitochondria Dilated ER Lysosomal storage Lipid accumulation Cytoplasmic accumulation of glycogen Hypoxic vacuoles Introduction to Proteins Part 1 Buffers resist change in pH; a good example is the bicarbonate system in the blood L isomer predominant in nature; counterclockwise NH3, COOH, R group, H R group classification of aa R group Classification Nonpolar, aliphatic R groups (hydrophobic) Aromatic R groups Aliphatic hydroxyl R groups (polar aa) Aliphatic sulfydryl (thiol) R group (polar aa) Basic R groups Acidic R groups (-charge at pH 7) aa Gly, Ala, Val, Leu, Iso, Met, Pro Phe, Tyr, Trp Ser, Thr Cys Lys, Arg, His Asp, Glu, Asn, Gln Plasma Membrane 1 Proteins define the unique functions of membranes, while lipids form the permeability barrier and define the basic architecture. Membrane Assymetry: Exoplasmic is choline-containing Cytoplasmic is amino containing Introduction to Proteins 2 Disulfide bonds are the most common covalent modification (formation of disulfide bridges). Disulfide bonds are formed by the oxidation of –SH side groups of a pair of cysteine residues to S-S linkage to form cystine. MW= # aa X molecular weight (in Da) Average molecular weight of aa=110 Da(.11kD) Cell Structure and Function 3 major structures found in cytoskeleton=actin filaments, intermediate filaments, microtubules Actin filamants are 15% of the total protein in the cell and consist of alpha, beta, and lambda classes ACTING BINDING PROTEINS Acting-Bindin Protein Fimbrin α-actinin Spectrin Filamin Dystrophin Function Binds actin filaments Binds membrane with fibroblasts, found in intestines RBC membrane inner surface Network structure; “leading edge” of moving cell Present in muscle cell; if defective=muscular dystrophy INTERMEDIATE FILAMENTS Inermediate Filament Acidic/Basic Keratins Distribution Epithelial cells Desmin, GFAP, vimentin Neurofilaments NFM, NFH) Lamins Muscle, glial mesenchymal cells (NF1, Neurons Proposed Function Tissue strength and integrity cells, Sarcomere organization, integrity Axon organization Nucleus Nuclear structure organization and The outer membrane of the nucleus is continuous with the RER, while the inner membrane of the nucleus is continuous with the nuclear lamins. Actin: Globular monomers of G-actin; 1 ATP per monomer; polymer is 2-stranded helix; 6 nm diameter Tubulin: globular dimers of alpha and beta that line up end to end; 1 GTP per dimer; polymer is hollow tube composed of 13 protofilaments NPC=nuclear pore complexes, composed of nucleophorin proteins Molecules larger than 60 kDa require other proteins which interact with nucleophorins for transport Plasma Membrane 2 Voltage gated ion channels: rely on selectivity filter and dehydration of ions Ligand-gated ion channels: ligand binding results in conformational change of receptor (energetically favorable) “Passive transport”: membrane transport mechanisms that transport molecules down the EC gradient, therefore not requiring energy “Active transport”: depends on energy input for function 3 types of active transporters: P-type, ABC-type, F & V type Blood Blood=6-8% of body weight Total blood volume: 5.5 L (6 quarts) RBC Volume=45% Average concentration of Hb: 13.6-17.2 g/dl (Men), 12.0-15.0 g/dl (Women) Erythrocyte count: 4.3-5.9 X 106 mm3 (Men), 3.5-5.0 X 106 mm3 (Women) Hematocrit: packed erythrocytes per unit volume in blood; 40-50% (Men); 35-45% (Women) Total erythrocytes: 5,000,000 per mm3 blood Total leukocytes: 7000 per mm3 of blood Total platelets: 250,000 per mm3 of blood LEUKOCYTES Cell Neutrophil Lifespan <1 week % 60-70% Eosinophil <2 weeks 2-4% Basophils 1-2 years <1% Lymphocytes Few months to several years 20-25% Monocytes Few days in blood, several months in connective tissue 3-8% Function Phagocytosis and destruction of bacteria Phagocytosis of antigen-antibody complex: destruction of parasites Similar to mast cells to mediate inflammatory response T cells: cell-mediated inflammatory response B cells: humorally mediated immune response Differentiate into macrophage; phagocytosis, presentation of antigen HUMORAL IMMUNITY Ig IgG IgM IgA Function Immunity against bacteria and viruses in extracellular fluid Immunity against bacteria and viruses in extracellular fluid Secreted by plasma cells in GI, Percent 80% 5% 15% respiratory tract, genitourinary tract, breast milk IgE Defense against multicellular parasites and allergic reactions IgD Fx not known T cells need AG presentation by MHC (II for helper, I for cytotoxic) <1% <1% MHC I found on all nucleated cells of the body MHC II found only on macrophages, macrophage-like cells, and B cells Epithelial Tissue Type of Tissue Simple squamous Simple cuboidal Simple columnar Pseudostratified columnar Stratified squamous keratinized Stratified squamous non-keratinized Stratified cuboidal Transitional (distended and non-distended) Gustatory Olfactory Stato-acustic Germinal Location Endothelium of blood and lymphatic vessels, mesothelium of peritoneal cavity Ducts of glands, covering of ovary, kidney tubules GI tract, gall bladder, parts of reproductive tract uterus, efferent ductules, part of respiratory tract bronchioles Most of respiratory tract, trachea, epidymus, nasal cavity Epidermis Wet inner surfaces, oral cavity, esophagus, vagina, conjunctiva of eye Ducts of sweat glands, pancreas, salivary glands Lines most of urinary tract Tongue Nasal passageway Covering inner ear Lines seminiferous tubules of testis The apical surface of epithelial tissue is capable of endocytosis, exocytosis, and transcytosis. Exocytosis occurs at the apical surface for exocrine glands and at the basolateral surface in endocrine glands. Distinguish between the terminal bar and the terminal web: Terminal web: where actin filaments embed at the base of the microvilli Terminal bar: place where two cells contact or attach to each other near the apical surface, located on the lateral domain of a cell; runs in a zone all around the circumference of a cell Note that cadherins are transmembrane proteins located in all three levels of the terminal bar. Gap junctions (nexus) have a low electrical resistance, so they are responsible for the peristaltic contraction of smooth muscle in the gut. Note that the basal domain serves to connect the epithelium to the underlying CT. Hemidesmosomes are in the basal domain, have attachment plaques on the cytoplasmic side, and contain integrin instead of cadherins. Desmosomes are located in the basolateral domain and contain cadherins, which are TM proteins that help link cells to adjacent cells. Type of Secretion Mucus Description Thick and viscous Serous Mixed Sebum Ceruminous Watery Serous/mucus Oily Waxy Mode of Secretion Merocrine Description Most common; excretion by exocytosis Secretion is pinched off of cell with part of cytoplasm and cell membrane lost Cell fills up with secretion, dies, and becomes the secretory product Whole living cell is the secretion Apocrine Holocrine Cytocrine Gland Goblet cell, sublingual salivary gland Pancreas, parotid, or sweat glands Salivary glands Sebaceous glands of skin Ceruminous glands of external auditory canal Gland Sweat glands, salivary glands, pancreas, goblet cells Lipid portion of milk of mammary gland Sebaceous gland of the skin Ovary, testis Introduction to Proteins 3 Beta-mercaptoethanol: reduces covalent disulfide bonds –S-S- (cystines) to sulfhydryls -SH + HS(cysteine); forms disulfide bridges 8M urea: disrupts noncovalent bonds; thought to weaken hydrophobic interactions and disrupt H bonds Protein folding is facilitated by: electrostatic interactions, disulfide bridges; thermodynamically favored Marginal stability of proteins is biologically advantageous Affinity chromatography: separates and purifies proteins based on that protein’s specific binding affinity for the molecule attached to the column matrix best method of protein purification b/c you can increase the yield of your protein of interest dramatically Connective Tissue Tissue Mesenchymal Mucous Loose (areolar) Dense irregular Dense regular collagenous Dense regular elastic Reticular Adipose Blood Cartilage Location Embryo and fetus Umbilical cord Loose packing around most organs and tissues, surrounds blood vessels Dermis, organ capsules, periosteum, perichondrium Tendons, ligaments, aponeurosis (tendonous extension) Ligamentum nuchae, flava Lymphatic tissue, bone marrow Omentum, subcutaneous fascia Cardiovascular, hematopoietic tissue Costal cartilage, trachea, pina, epiglottis Lipoprotein lipase makes white adipocytes larger because it breaks down fatty acids, VLDL, and chylomicrons to free fatty acids and glycerol, which are then taken up and stored by adipocytes as lipids; stimulated by insulin Insulin: acts on adipocytes to form triglycerides from glucose, increases uptake of glucose and production of lipoprotein lipase Hormone sensitive lipase makes white adipocytes smaller by breaking triglycerides down into free FA that complex with albumin the blood for transport around the body; stimulated by leptin, epinephrine, and norepinephrine Leptin: hormone produced by adipocytes that targets the hypothalamus. Decreases food intake and increases energy consumption. Epinephrine/norepinephrine: stimulate hormone sensitive lipase to mobilize fatty acids Thermogenin: mitochondrial membrane protein that permits the back flow of H+ instead of using them for ATP production; this uncouples the oxidation process and leads to heat generation in brown adipocytes Cell Type Fibroblast Fixed Mesenchymal Fixed Reticular Fixed Adipocytes fixed Chondrocyte Fixed Osteocyte Fixed Blood cells Fixed Macrophages Wandering Mast cells Wandering Plasma Cells Endothelial Smooth Muscle Cells Wandering Associated Associated Pericytes Associated Function Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Synthesis and maintain extracellular matrix of CT Phagocytic cells, antigen presenting cells Store mediators of the immune response Produce antibodies Line the blood vessels Surrounds the endothelium and controls vessel size Multipotential for vessel growth and repair As a monocyte leaves the blood vessel and travels to the extracellular matrix, it becomes a macrophage. Macrophages derive from monocytes (from a cell line called granulocytic-mononuclear stem cell) but monocytes/macrophages are in fact agranulocytic. Granules of mast cells contain, among other things, histamine (too much causes anaphylaxis) and leukotrienes (which cause release of cytokines) Remember that collagen accounts for 20% of the protein in the body and 30% of the dry weight of the body. Remember: Hydroxylysine holds together the three α-polypeptide chains to form tropocollagen, and fibrils are held together by hydroxyproline, which binds the tropocollagen together. TYPES OF COLLAGEN Type I Function Resists tension; forms thick fibers II Resists pressure; forms thin fibers Forms structural framework of spleen, liver, lymph nodes, smooth muscle, adipose tissue Does not form fibers; forms meshwork of the lamina densa of the basal lamina to provide support and filtration III Aka reticular fibers IV Location Dermis, tendon, ligaments, capsules of organs, bone, dentin, cementum Hyaline cartilage, elastic cartilage Lymphatic system, spleen, liver, cardiovascular system, lung, skin Basal lamina Collagen Formation: Collagen is transcribed in the nucleus and translated in the ER, where Pro and Lys are hydroxylated, procollagen is formed and then secreted via the Golgi. Procollagen peptidase: cleaves procollagen to tropocollagen Lysyl oxidase: links hydroxylysine molecules of adjacent tropocollagen molecules to form fibrils Distinguish between Ehler’s Danlos Type VII (due to procollagen peptidase change) and Ehler’s-Danlos Type IV (deficiency in Type III collagen) Remember that elastic fibers= elastin + microfibrils. Elastin contains the unique enzymes desmosine and isodesmosine. Elastic fibers are formed like collagen fibers, but lysyl oxidase links tropoelastin (instead of tropocollagen) to form elastin (instead of collagen). Two other types of elastic fibers exist: oxytalan fibers and eulanin fibers. Aggrecan is a free proteoglycan whose core protein is chondroiton sulfate and keratin sulfate. An aggrecan aggregate is HA with hundreds of aggrecan molecules. It is found in cartilage and is much more gel-like and compression resistant. PROTEOGLYCANS Proteoglycan Type Core Protein Location Aggrecan Free Perlacan Syndecan Free Transmembrane Fibroglycan Transmembrane Chondroiton sulfate and keratin sulfate Heparin sulfate Heparin sulfate and chondroiton sulfate Heparin sulfate Cartilage matrix (compression resistant) Basal lamina One end in cytoplasm and the other end in the EMC Binds where there is collagen and fibronectin (in basal lamina) GLYCOPROTEINS Type of Glycoprotein Fibronectin Location Basal lamina Laminin Entactin Tenascin Chondronectin Osteonectin Basement membrane Basement membrane Basement membrane Cartilage matrix Bone matrix Binds to: Heparin sulfate and collagen type IV collagen IV and heparin sulfate Laminin and collagen IV Syndycan and fibronectin Cell integrin to collagen II Cell integrin to collagen I Remember that basement membrane is a light microscope term, is located beneath the basal surface epithelia, and has 3 layers at the EM level. Basal lamina is an EM terms and there are two views as to what composes it. Dr. Crissman and our text present that the basal lamina consists of the lamina lucida and the lamina densa. LAYERS OF THE BASAL LAMINA Layer Lamina lucida Lamina densa Location Next to cell membrane Fuzzy band below lamina lucida Lamina reticularis Below lamina densa Contains: Integrins, laminins, and entactin Collagen IV, perlacan (heparin sulfate) and fibronectin Reticular fibers (collagen III), collagen IV and VII of anchoring fibrils Remember that the basement membrane acts as a macromolecular sieve. Myoglobin and Hemoglobin O2 is not very soluble in water, Mb helps with this Mb increases O2 availability in muscles Hb carries O2 from capillaries in lungs to capillaries in tissues N=hill coefficient; a measure of the degree of cooperativity N<1 negative cooperativity, N>1 positive cooperativity, N=1 no cooperativity In the Monod model, the T and R forms are in equilibrium. T state and O2 release is favored when O2 is low. R state and O2 binding is favored when O2 is high (O2 binding stabilizes the R state). O2 affinity increases as fractional saturation increases. In the Koshland model, the protein is in either the T or the R form with no ligand bound. The ligand binding to one subunit can cause a conformational change in a neighboring subunit. The general model combines features of Monod and Koshland. The Monod model describes Hb binding. Homotropic effectors: bind to the same type of site as the ligand (ex: O2 binding to Hb) Heterotropic effectors: bind to a site different from the ligand, but still induces a conformational change Examples: H+ binds to His to stabilize the T state and reduces the affinity for O2. Salt bridges form when His 146 is protonated, stabilizes the T (low affinity) state. Known as the Bohr effect. 2,3-BPG binds to the center of Hb, stabilizes the T state and reduces the affinity for O2. Hb binds one molecule of BPG, which reduces the affinity for O2 BPG is negatively charged and is surrounded by positive charge when it binds to Hb CO2 reacts with the N-termini to stabilize the T-state and reduces the affinity for O2. N terminus can carry CO2 and carbamino Val forms a salt bridge with Arg to stabilize T state. O2 binding promotes the release of CO2 from the N termini. High concentrations of O2 in the lungs result in saturation of Hb with O2, ligation of the O2 binding site reduces the affinity for CO2 and promotes CO2 release (Haldane effect). Cl binding reduces affinity for O2. Chloride binds to the deoxy form, decreasing O2 affinity Forms a salt bridge between Arg on one subunit and N-terminus of another subunit. HB EFFECTORS Ligand Effect on O2 affinity Effect of binding O2 BPG Increased Reduces CO2 Reduced Protons Reduced Homotropic Heterotropic effector, stabilizes the T state Heterotropic effector, stabilizes the T state Heterotropic effector, stabilizes the T state O2 affinity increases as temperature decreases. At lower temperatures, there is not a very high oxygen requirement. CO is toxic because it binds with a higher affinity to O2 site in Hb, so it physically blocks the O2 site and causes higher O2 affinity so it can’t release O2 either. It also binds to cytochrome oxidase and myoglobin. Cells in sickle cell disease aggregate when Val residues insert into hydrophobic pockets of another HbS molecule, which leads to polymerization and aggregation and long fibers which cause RBC sickling. TCA Cycle “Waltz around the cycle” -α-Ketoglutarateglutamate Oxaloacetateaspartate Citrateacetyl-CoAfatty acids Succinyl CoAheme synthesis Cell Motility Two Phases: Extravasation and Diapedesis Extravasation consists of 1)Activation of endothelial cells 2)Trapping 3)Adhesion 4)Migration through wall Diapedesid consists of 1)extension of lamellapodia 2)adhesion by focal attachments to adhesion molecules and collagen fibers in ECM 3)cytoplasm flows forward 4)retraction with footprint Laminin: regulates neural outgrowths Fibronectin: promotes migration Cell Cycle Phase of cell cycle G1 N 2n Time 6-12 hours S G2 Mitosis 4n 4n 6-8 3-4 1 hr Cancer cells have a different amount of genetic material than a diploid cell. Cell cycle is regulated by: 1. 2. 3. 4. 5. 6. 7. 8. Mechanical force (stretching) Injury to tissue (ischemia) Cell death Growth factors or mitogens Clock or timer (correct order of event) Mechanism to ensure that each event is triggered only once per cycle Completion of each step Rate of progression ****Phosphorylation of p53 inhibits the cell cycle, while dephosphorylation of Rb inhibits the cell cycle p16 helps prevent cancer by cellular senescence=tumor suppression mechanism in which the levels of p16 increase after a certain number of cell divisions to cause cell death; normal in aging but not a good thing in cancer (i.e. in stem cells or beta cells in pancreas) Loss of p53 allows cells with damaged DNA to survive and divide, thereby propagating potentially oncogenic mutations Enzymes Enzymes have a higher affinity for the TS than for the substrate. Catalysis works by: proximity, strain or distortion of a bond, acid-base catalysis, or nucleophilic catalysis. Nucleophilic catalysts form a covalent complex with part of the substrate. Nucleophilic groups (O of serine OH, S of Cys, N of Lys or His, COO-of Glu and Asp) Chymotrypsin: hydrophobic residue of the substrate binds in the hydrophobic pocket on the enzyme, His is in proximity with Ser and withdraws a proton (acting as a general base catalyst), Ser now a good nucleophile, Asp H bonded to His and orients it with respect to Ser, Ser covalently binds with the carbonyl carbon of the substrate, c terminal peptide fragment is released, water hydrolyzes this VITAMINS AS PRECURSORS Vitamin Cofactor Reaction Thiamin TPP aldhehyde Group transfer Riboflavin FMN or FAD Electron/H transfer Niacin NAD or NADP Electron/H transfer Pantothenic Coenzyme A Acyl-group transfer Pyridoxine PyrodoxalP Carboxyl transfer Biotin Biocytin One-carbon reactions Vit B12 Coenzyme B12 1,2 shifts of hydrogen Lipoic acid Lipolysine H and acyl transfer E-S formation is fast compared to E-S to product and enzyme, so it is the rate-determining step. Michaelis-Menten Equation: 𝑉 = 𝑉𝑚𝑎𝑥•[𝑆] 𝐾𝑚+[𝑆] Km=dissociation constant Briggs-Haldane: Km is NOT a dissociation constant but it is a kinetic term. Double reciprocal plot: can tell ratio of Km to Vmax, 1/Vmax (y intercept), Km (x intercept) Competitive Inhibition: Km changes but Vmax does not Noncompetitive Inhibition: Km stays the same but Vmax changes Turnover number=Kcat; number of substrate molecules converted per molecule of enzyme per unit of time