The Radiation Balance
advertisement

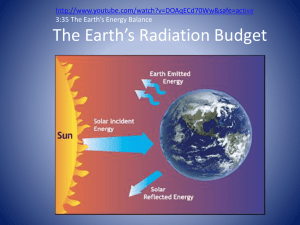
The Radiation Balance The Spectrum of Electromagnetic Radiation The incoming energy from the Sun to Earth is mainly visible sunlight, called the visible portion of the spectrum of electromagnetic radiation. We perceive visible sunlight as colors from violet (shortwave radiation) to red (long-wave radiation). The sequence of colors seen in the rainbow represents the spectrum of this light, ordered according to wavelength. A relatively minor amount of energy leaves the sun as radiation with shorter wavelength (ultraviolet) and as radiation with longer wavelength (infrared or heat radiation). Visible light (the colors of the rainbow) occupies the narrow part of the spectrum between the dashed lines in the first figure. The (invisible) light with wavelengths just shorter than violet is called ultraviolet, meaning beyond violet. It is largely absorbed in the atmosphere and only a modest amount of this light arrives at the surface of Earth. This is fortunate, because ultraviolet light, abbreviated UV, can cause damage to skin and to vertebrate retinas and interferes with photosynthesis in algae and plants. Protection from UV light is provided (among other things) through the ozone layer in the lower stratosphere. The (invisible) light with wavelengths just longer than red is called infrared, meaning below red and often simply referred to as IR. IR is heat radiation coming from the Sun. Interestingly, some organisms, especially some insects, can see UV and navigate by it, while some snakes have adapted to see in IR. The Sun’s Radiation All objects (unless they have a temperature of zero degrees Kelvin) radiate energy. The temperature of an object determines the type of radiation it emits. Hence, every star radiates energy with wavelengths corresponding to its surface temperature: a cooler star would radiate a more reddish light, a hotter one a more bluish one. Reddish and bluish stars can be seen readily in the night sky. Most of the light emitted by our star, the Sun, is yellowish. By measuring the light received from the Sun we know that its radiation corresponds to a surface temperature of about 6000°C (or 6300 K where K is the symbol for units of Kelvin). The organisms on Earth have long adapted to the nature of this sunlight. Blue-green light penetrates most deeply into the sea, so visual acuity of deep-living fishes is greatest in the blue-green part of the spectrum. Our own eyes are specialized for yellow, green and red (the colors of traffic lights). Plants use mostly red light to grow and reflect the rest, making them appear green. The nuclear fusion generator that powers the Sun is deep in the Sun’s center (called the core of the Sun), hidden by a thick layer of hot hydrogen and helium. This is fortunate for us, because no one could look at the Sun's power plant and survive the experience: the temperature is near 15 million degrees of Kelvin. The reason the power plant does not blow the sun apart is that the enormous pressure of the solar matter surrounding it prevents it from doing so. Conversely, the Sun does not collapse because of the counter-pressure generated in the core because in the Sun the gravitational and radiative pressures are in balance. It takes about a million years for the energy to made in the core to reach the surface of the Sun. From there it takes less than 10 minutes at the speed of light to reach Earth. The energy the core generates comes from the fusion of hydrogen nuclei to make helium nuclei. Through this process some of the mass is lost and reappears as energy (according to the famous equation of Albert Einstein E=mc2), resulting in the loss of 4.5 million tons of mass from the Sun each second. Not to worry however: there is still plenty of hydrogen to burn about two-thirds of the mass of the sun consists of hydrogen and the process has been going on for some 5 billion years and will do so in the future for about as long (Also see the Glossary entry on Solar constant.). The Earth’s Radiation Just as the temperature of the Sun's surface determines the kind of electromagnetic radiation it delivers, so the temperature of the Earth determines what kind of radiation it puts out to space, which turns out to be infrared, or heat radiation. As mentioned, the amount of heat Earth has to get rid of is entirely determined by the amount it receives from the Sun in the first place minus the portion it immediately reflects back into space. (The reflected portion cannot be included in the portion that is reradiated because it does not actually heat the Earth. However, this reflected portion is visible from a spacecraft or when standing on the Moon: the Earth is reasonably bright as planets go, mainly because of its clouds and its ice caps and reflects 30% of the light it receives back to space. This proportion, called the “albedo” of Earth, is less than that reflected by Venus, but more than that of Mars. The kind of infrared radiation given off by the various areas of Earth's surface depends on their temperature, which in turn depends on a number of factors such as the amount of sunlight absorbed and the heat spent in evaporating water. In the desert, after sundown, one can readily sense the highenergy infrared given off by rocks recently warmed by the Sun's rays, but all surfaces radiate heat, whether recently warmed by the Sun or not. Typically, temperatures on the surface of Earth vary somewhere between freezing and 90°F, which roughly defines the broad "spectrum" of infrared radiation emitted upward into the atmosphere. The Absorption of IR by Greenhouse Gases Now that we have explained the relationship between the radiation emitted by an object and its temperature, we can explain how greenhouse gases warm the Earth. Certain lines within the electromagnetic spectrum, specifically certain wavelengths of infrared radiation, have precisely the right energy to interact with certain molecules present within Earth’s atmosphere. When such a special packet of light (called a photon) interacts with the appropriate molecule, the molecule absorbs the energy, and increases its temperature accordingly. It then re-radiates heat to its surroundings. When measured with an instrument, this absorbed heat forms absorption lines or even absorption bands that are broader than lines and may include several lines. The absorption bands of different greenhouse gases may or may not overlap with each other. When a greenhouse gas is very abundant the absorption lines for which it is active are said to become saturated, that is, most of the available IR will have been absorbed by the molecules of that gas. Adding more of that gas will not absorb more IR in the proportion of the addition. For example, many of carbon dioxide’s absorption lines are fairly well saturated. This is the fundamental reason that the 30 percent increase in carbon dioxide since the industrial revolution has not increased the background greenhouse effect by 30 percent. Only a doubling of CO2 will have a substantial effect, through the amplification caused by water vapor (resulting in a 4 to 6°F increase, according to the best estimates). Another doubling on top of this presumably will have a similar effect, in part through a broadening of the absorption lines affected. Other greenhouse gases that were once rare or even nonexistent are now being introduced vigorously by human activities, and they can take up new absorption lines that have not been previously occupied. Thus, their effect on interception of IR and the associated greenhouse effect is accordingly enhanced. Methane is such a gas, as are the CFC’s formerly used in refrigeration.