Physics 535 lectures notes: 1 * Sep 4th 2007
advertisement

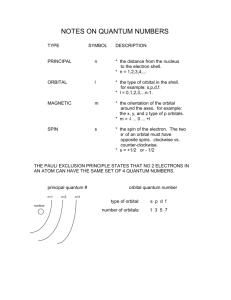
Physics 249 Lecture 22, Oct 26th 2012 Reading: Chapter 7.6, 7.7, 7.8, then 7.4(magnetic moment) and 7.5 Homework due today. New HW available on web site due Nov 2nd 1) Multi-electron atoms In general as you progress to multi-electron atoms the electrons will fill orbitals progressing through m, ms, l, n quantum numbers. We discussed in the previous lecture the filling of ms and m levels with the m levels with spins aligned in one direction filling first and then the second set of m levels with spins in the opposite direction. The primary exception. A 4s state is at lower average radius than a 3d state. This is because of the three additional maxima at lower radius. The 4s state will be shielded from less of the nuclear charge than the inner 3d state effectively increasing the effective Z in the energy formula. Zee is large enough that it counteracts the fact that n=4 rather than 3 in the energy formula for the orbital giving a larger magnitude negative energy. Since the 4s state is lower energy it fills first. The ionization energy of potassium, K 19, which has the first 4s orbital is 4.407 2 2 𝑍𝑒𝑓𝑓 𝐸1 𝑍𝑒𝑓𝑓 ∗ 13.6 𝐸𝑛 = − = − = −4.407 𝑛2 42 𝑍𝑒𝑓𝑓 = 2.28 rather than 19! The d orbital must have a Zeff smaller than a certain value to give higher energy (smaller negative energy). 𝐸𝑛 = − 2 2 𝑍𝑒𝑓𝑓 𝐸1 𝑍𝑒𝑓𝑓 ∗ 13.6 = − = −4.407 2 𝑛 32 𝑍𝑒𝑓𝑓 < 1.71 Note that perfect shielding would give Zeff = 1. It is interesting to compare this to Argon, Ar 18, a 3p orbital right before the jump to a 4s orbital with an ionization energy of 15.7576 𝐸𝑛 = − 2 𝑍𝑒𝑓𝑓 𝐸1 𝑛2 =− 2 𝑍𝑒𝑓𝑓 ∗13.6 32 𝑍𝑒𝑓𝑓 = 3.23 rather than 18 = −15.7596 Compared to the 3d orbital or the 4s orbital of the next energy level the 3p orbital has a two maxima with substantial probability at low radii that exposes it to much more unshielded nuclear charge. There is a fairly simple system of figuring out the order of n and l orbital filling. In the following triangle draw diagonal lines up from left to right. The orbitals fill in that order. Minor exceptions. There are some minor exceptions even to these rules in larger atoms. For instance sometimes there is some alternating between n+1 s and n d orbits. For reference radial wave functions 2) The period table. The completion of the filling of a series of orbitals, or subshells, before the start of the s orbital of the next energy level is called closing of a shell. The organization of periodic table can be understood from these principles. The blocks correspond to types of orbitals and the end of a row indicates the completion of a shell or subshell (in cases of changes in order between s and other orbitals) before the start of a new s orbital. Atoms in columns share chemical properties because their outer elections are from the same position in a give orbital. 3) Properties of atoms: Atomic bonding Atomic bonding can be understood based on the shapes orbital configurations of the atoms. The most important factor is the radial wave functions, which determine the orbital/ionization energies. a) Ionic bonding For instance the low ionization energy of lithium, sodium, potassium … makes their outer electron available for molecular bonding. If another atom can add an extra electron into high ionization energy orbital, for instance a final empty p orbital as in chlorine or florine, the overall energy of the system will be lowered. The same patterns of ionization potential will reoccur around each new s type orbital transition. The changes in ionization energy are quite dramatic at those points. b) Covalent bonding. Also in some systems two atoms can share an electron partially closing the shell of both atoms and lowering the energy of the entire system. Partially because the probability distribution is shared with ~1/2 for each atom.