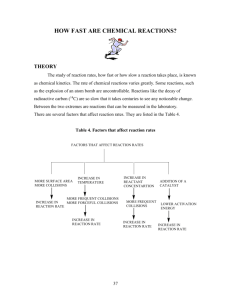
Flame Tests When elements are heated to high temperature, they
advertisement

Flame Tests When elements are heated to high temperature, they may enter an excited state. In an excited state, the electrons move to higher energy levels. The changes in energy that occur when the excited electrons return to their ground state cause the substance to be luminous or emit light. The observed colors of spectrum of the substance is caused by the set of visible wavelengths of light emitted. Since each element emits a unique set of wavelengths, emission spectra can be used as a tool to identify the elements. One method used to demonstrate the emission spectrum of a substance is the flame test. Using this method, a small amount of a substance is heated and the characteristic glow of the substance is observed. In this experiment you will perform a flame test on several metallic salts. Based on your observations, you will develop a reference tale which lists the flame color for each metal ion. You will then perform a flame test on an unknown substance. By comparing your observations to the data in your reference table, you will be able to identify the components of a metallic salt mixture. Objectives: Safety demonstrate designated laboratory techniques. Observe spectra emitted from selected ions. Evaluate the usefulness of this method of metal identification. Pre-lab Questions: These questions must be answered before the lab may be performed, and written in the write up between objective and materials. 1. Practice safely lighting and turning off a burner. 2. What happens to the electron in an excited state? 3. Which ion of a solution can be identified by the color emitted? Equipment: goggles and aprons nichrome (or Pt) wire loops Bunsen burners Cobalt glass squares 0.1 M solutions of LiCl, CaCl2, SrCl2, BaCl2, MnSO4, FeSO4, FeCl3, CoCl2, Pb(NO3)2, NaCl, KCl Safety: goggles and aprons need to be worn at all times. Do not move the materials to any other station. Make sure you are observing the color of the solution, not the regular color of the flame. Alert the teacher before relighting any burner. Procedure: 1. Prepare a data table as directed. Goggles and aprons need to be worn at all times! 2. Dip the clean wire loop into an ion sample at your station. Observe the color and record. 3. Repeat step 2 at each station 4. Repeat step 2 at unknown station 5. Repeat step 2 at station of Na+ and K+ ion mixture, then observe the mixture again through the Co glass. Data/Analysis: metal ion name lithium ion with charge Li+ flame color calcium strontium barium manganese II iron II (FeSO4) iron III (FeCl3) cobalt II copper II lead II sodium potassium unknown sodium and potassium mix color of mixture through cobalt glass Conclusion: 1. What ion would you predict to be contained in the sample of the unknown? Why? 2. Based on the results and observations, would this method be practical to determine metals in a mixture? If not, why not? 3. What difference did you see observing the flame of: a. sodium vs. sodium and potassium mixture b. potassium vs. mixture 4. What was the purpose of using the cobalt glass to observe the sodium and potassium mixture? 5. Give at least two reasons why the flame test is sometimes invalid. 6. What other means of qualitative analysis can be used to identify metals?