Initial Flow of Water
advertisement
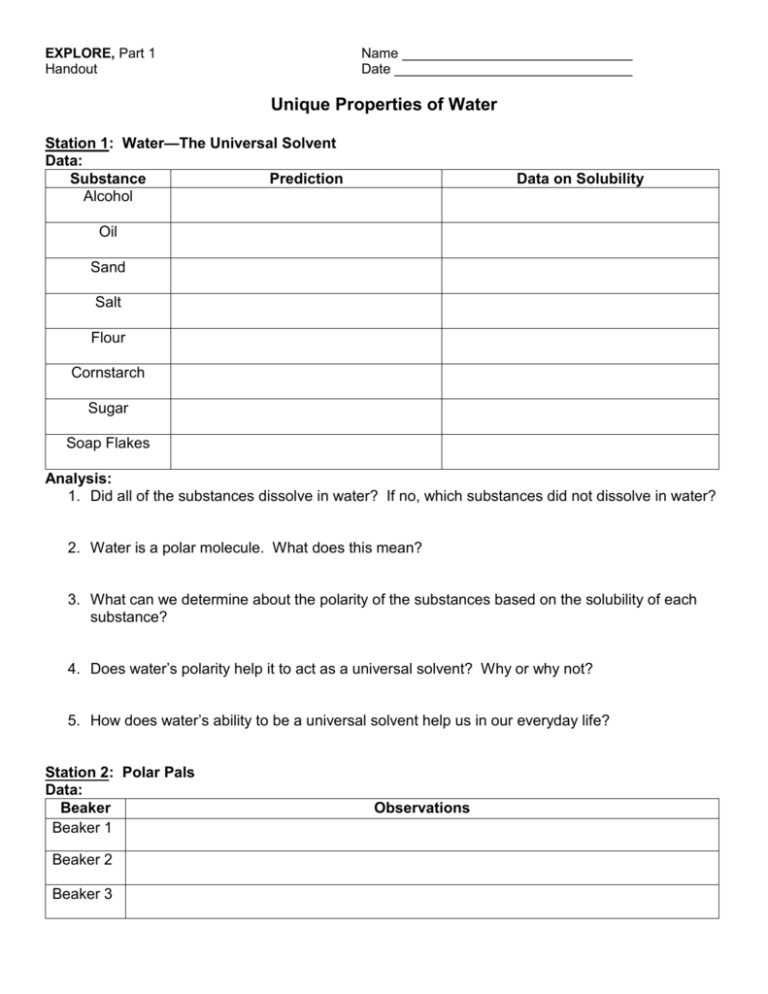
EXPLORE, Part 1 Handout Name ______________________________ Date _______________________________ Unique Properties of Water Station 1: Water—The Universal Solvent Data: Substance Prediction Alcohol Data on Solubility Oil Sand Salt Flour Cornstarch Sugar Soap Flakes Analysis: 1. Did all of the substances dissolve in water? If no, which substances did not dissolve in water? 2. Water is a polar molecule. What does this mean? 3. What can we determine about the polarity of the substances based on the solubility of each substance? 4. Does water’s polarity help it to act as a universal solvent? Why or why not? 5. How does water’s ability to be a universal solvent help us in our everyday life? Station 2: Polar Pals Data: Beaker Beaker 1 Beaker 2 Beaker 3 Observations Analysis: 1. Which substance was soluble in water? Why? 2. Which substance was insoluble in water? Why? 3. Did the liquids in the last beaker mix? Why or why not? Station 3: Sinkin’ Lincoln Data: Substance Prediction of Number of Drops before Overflow Actual Number of Drops Before Overflow Plain Water Soapy Water Sketch: Analysis: 1. Surface tension is created by forces between molecules. Are the forces cohesive or adhesive? Explain your answer. 2. What did the soap do to the surface tension of the water? Explain your answer. 3. How does surface tension explain the pain a swimmer feels when he jumps off the diving board and does a belly buster? Station 4: Passengers in a Boat Data: Number of Mass of Mass of boat pennies needed empty boat and pennies to sink boat Volume of boat Density of empty boat Density of boat with pennies Sketch: Analysis: 1. What is buoyancy and how does it relate to this lab? 2. Does the mass of the boat affect the buoyancy? Why or why not? 3. How are the concepts of buoyancy and density used to design boats that will float on water? Station 5: A Density Column Data: Object Wooden Object Observations Plastic Object Aluminum Ball Sketch: Analysis: 1. If water’s density is 1.0 g/mL, what can be determined about the other liquids’ densities in the column? 2. Rank the densities of the liquids and the solids from most dense to least dense. 3. Using this information, explain how the Titanic could float on water. Station 6: Capillary Action Data: Beaker 1 Observations 2 3 Analysis: 1. Is capillary action a cohesive or adhesive force? Explain your answer. 2. Which liquid experienced the greatest capillary action? Explain your answer. 3. How do plants use capillary action to acquire water? Station 7: Go With the Flow Data: Flow of Water Observations Initial Flow of Water Flowing Together Flowing Separating Analysis: 1. Which direction does the water flow when you first take the tape off the bottom of the can? 2. What causes the attraction of the water to begin flowing together? 3. What allows the water to continue to flow in one stream?