PHYL 423 - WordPress.com
advertisement
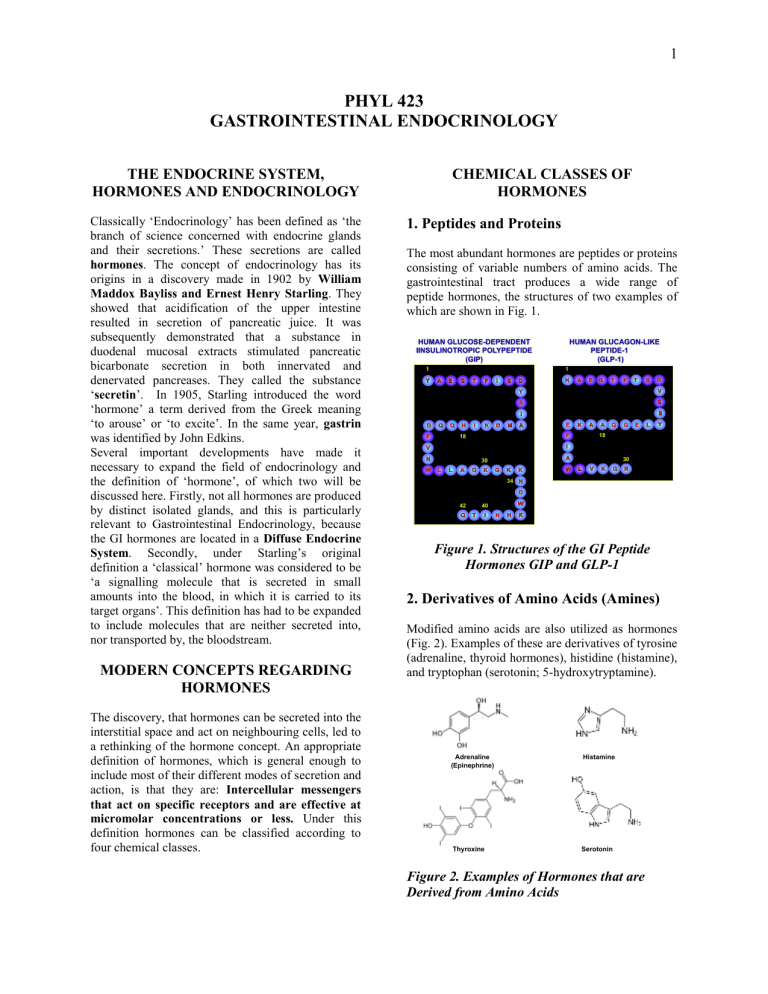
1 PHYL 423 GASTROINTESTINAL ENDOCRINOLOGY THE ENDOCRINE SYSTEM, HORMONES AND ENDOCRINOLOGY Classically ‘Endocrinology’ has been defined as ‘the branch of science concerned with endocrine glands and their secretions.’ These secretions are called hormones. The concept of endocrinology has its origins in a discovery made in 1902 by William Maddox Bayliss and Ernest Henry Starling. They showed that acidification of the upper intestine resulted in secretion of pancreatic juice. It was subsequently demonstrated that a substance in duodenal mucosal extracts stimulated pancreatic bicarbonate secretion in both innervated and denervated pancreases. They called the substance ‘secretin’. In 1905, Starling introduced the word ‘hormone’ a term derived from the Greek meaning ‘to arouse’ or ‘to excite’. In the same year, gastrin was identified by John Edkins. Several important developments have made it necessary to expand the field of endocrinology and the definition of ‘hormone’, of which two will be discussed here. Firstly, not all hormones are produced by distinct isolated glands, and this is particularly relevant to Gastrointestinal Endocrinology, because the GI hormones are located in a Diffuse Endocrine System. Secondly, under Starling’s original definition a ‘classical’ hormone was considered to be ‘a signalling molecule that is secreted in small amounts into the blood, in which it is carried to its target organs’. This definition has had to be expanded to include molecules that are neither secreted into, nor transported by, the bloodstream. MODERN CONCEPTS REGARDING HORMONES The discovery, that hormones can be secreted into the interstitial space and act on neighbouring cells, led to a rethinking of the hormone concept. An appropriate definition of hormones, which is general enough to include most of their different modes of secretion and action, is that they are: Intercellular messengers that act on specific receptors and are effective at micromolar concentrations or less. Under this definition hormones can be classified according to four chemical classes. CHEMICAL CLASSES OF HORMONES 1. Peptides and Proteins The most abundant hormones are peptides or proteins consisting of variable numbers of amino acids. The gastrointestinal tract produces a wide range of peptide hormones, the structures of two examples of which are shown in Fig. 1. HUMAN GLUCOSE-DEPENDENT IINSULINOTROPIC POLYPEPTIDE (GIP) 1 1 Y HUMAN GLUCAGON-LIKE PEPTIDE-1 (GLP-1) A E G T F I S D H A E G T F T S V S S S I D Q Q F H I K D M A E K A F 18 A Q G E L Y 18 I V N W D Y A 30 L L A Q K G K K W 30 L V K G R 34 N D Q W 40 42 T I N H K Figure 1. Structures of the GI Peptide Hormones GIP and GLP-1 2. Derivatives of Amino Acids (Amines) Modified amino acids are also utilized as hormones (Fig. 2). Examples of these are derivatives of tyrosine (adrenaline, thyroid hormones), histidine (histamine), and tryptophan (serotonin; 5-hydroxytryptamine). Adrenaline (Epinephrine) Histamine Thyroxine Serotonin Figure 2. Examples of Hormones that are Derived from Amino Acids 2 inflammatory actions and suppresses tumor growth. Adrenaline is not strictly a GI hormone since its major site of synthesis is the adrenal. However it is important in the regulation of GI blood flow during stress. Thyroid hormones (Thyroxine (T4); Triiodothyronine (T3)) are produced by the thyroid. However the GI tract both acts as a reservoir for thyroid hormone storage and, as in other tissues, actively converts T4 to the major active form, T3. The major role of thyroid hormones in the gut is probably regulation of cell metabolism. Histamine is a central molecule in the regulation of acid secretion and serotonin is involved in the regulation of gastrointestinal motility and secretion. Figure 4. Structure of a Prostaglandin and Platelet Activating Factor 3. Steroids Steroids are derived from cholesterol. A number of steroids have defined actions on the gastrointestinal tract. For example, progesterone and estradiol modulate GI motility and cortisol influences GI tract development, Aldosterone is involved in the regulation of ion and water transport. Progesterone Estradiol Until recently, nutrients were excluded from the definition of hormone. However, 'orphan' G ProteinCoupled Receptors (GPCRs) that bind fatty acids have now been identified (Fig. 5) in the intestine (enteroendocrine cells & mast cells) and in pancreatic islets (beta cells). These receptors are coded for in a gene cluster and exhibit different responsiveness to fatty acids of different chain length. Human Chromosome 19q13.1 CD22 Glucocorticoids Cortisol GPR40 GPR41 GPR42 Aldosterone Receptor Figure 3. Examples of Steroids With Gastrointestinal Functions Although most steroids are synthesized outside of the GI tract, the liver and intestine contain high concentrations of several isoforms of the enzyme 17 -hydroxysteroid dehydrogenase and they are probably major sites of inactivation of steroids. 4. Fatty Acids The major fatty acid derivatives are members of the eicosenoid family, which includes the prostaglandins, leukotrienes, thromboxanes, and prostacyclin, and platelet-activating factor (Fig. 4), which is a phosphatidylcholine-like molecule. Various members of the prostaglandin family have been shown to regulate GI secretion and motility, and to have protective effects on the GI mucosa. PAF has anti- Specificity GPR40 Medium to Long Chain FAs GPR41 Short Chain FAs GPR43 Short Chain FAs Figure 5. The GPR40-43 Family GPR43 3 synthesized in cells that are distributed diffusely throughout the stomach and intestines. When hormones, such as oxytocin and vasopressin, are secreted by neurons into the bloodstream, the mode of signal transmission is referred to as neuroendocrine secretion. It is not thought to be a normal mode of transmission by GI hormones. HORMONE-TARGET RELATIONSHIPS IN THE GASTROINTESTINAL TRACT The following terms have been introduced into the literature to describe the manner in which the various hormones act on their target tissues. Examples that are of relevance to the functioning of the gastrointestinal tract will be considered. C. Paracrine In paracrine secretion the hormone (parahormone) is released into the extracellular space and diffuses to neighbouring cells within a fixed area of influence (Fig. 6C). Some of these locally acting parahormones, for example somatostatin, may additionally act in an endocrine manner. Some texts also refer to neurotransmission (release of transmitters from neurons) as paracrine secretion. A. Endocrine and B. Neuroendocrine This refers to the ‘classical’ secretion of a messenger into, and transport by, the bloodstream (Figs. 6A and 6B). Outside of the GI tract, many hormones are synthesized in discrete glands, growth hormone in the pituitary and insulin in the endocrine pancreas (islets of Langerhans) for example, but the GI hormones are A. ENDOCRINE (E.G. INSULIN) A. ENDOCRINE (E.G. INSULIN) Secretory Granule ...... . ...... ......... Hormone ... .. . ... . . . . . . . . .. ... . . Receptors B. NEUROENDOCRINE OXYTOCIN) B.(E.G. NEUROENDOCRINE (E.G. OXYTOCIN) C. PARACRINE (E.G. SOMATOSTATIN) D. AUTOCRINE (E.G. GROWTH FACTORS) ........ .. . ........ ... ..... ..... .. .. . . ........ .. . ........ .... ..... . .. . . . . .. . E. JUXTACRINE (E.G. EGF) F. INTRACRINE (E.G. STEROID HORMONES T4/T3) . .. . G. EXOCRINE (E.G. EGF) Figure 6. Hormone-Target Relationships . . . . . . .. . .. 4 D. Autocrine As with paracrine secretion, the hormone is secreted into the extracellular space, but in this case it acts on its cell of origin (Fig. 6D). Many growth factors act in this way, and their overproduction can lead to tumor development. Examples of GI hormones signaling by endocrine, paracrine and autocrine pathways are shown in Table 1. Mode of Communication Endocrine Paracrine Autocrine Type of Regulator Hormone Parahormone Autocrine Example Secretin Somatostatin IGF-1 Table 1. Examples of Hormones E. Juxtacrine In some cases the hormone is synthesized as a membrane bound precursor molecule. This may be cleaved to produce a soluble molecule or remain attached to the plasma membrane where it can influence neighbouring cells. When it remains attached, its mode of action is termed juxtacrine (Fig. 6E). One example is epidermal growth factor (EGF). F. Intracrine Some hormones are synthesized within the cells in which they act. For example, steroid hormones can be produced by their target cells and thyroxine (T4) is secreted by the thyroid gland but metabolized to the more active form, triiodothyronine (T3) by intracellular enzymes in cells in the periphery, including the GI tract. This form of signal transmission is termed intracrine (Fig. 6F). G. Exocrine A few signalling molecules are secreted into the lumen of a gland and are resistant to proteolytic enzymes which are also secreted. EGF, for example, is produced by the salivary glands and can act within the stomach. The Trefoil Peptides and the antibacterial defensins are components of the innate immune system. H. Pheromones Chemicals released by an organism into the environment that alter the behaviour or gene expression of another organism of the same species are referred to as pheromones. They have been extensively studied in lower species, such as insects, where they act as sex attractants, and they probably serve a similar role in humans. Many mammals have anal glands that secrete pheromones. THE DIFFUSE ENDOCRINE SYSTEM AND ENTEROENDOCRINE CELLS The term APUD (Amine Precursor Uptake and Decarboxylation) cells was originally introduced to describe cells exhibiting co-localization of biologically active peptides with specific amines and their synthesizing and processing enzymes. It is now more common to call them enteroendocrine cells. They are distributed throughout the GI tract, hence the combined system is termed the Diffuse Endocrine System. Although the cells are scattered in the gut mucosa, where they represent approximately 1% of the epithelial cell population, they constitute one of the largest populations of hormone-producing cells in the body. Some of the enteroendocrine cells are of an open type with microvilli that sense changes in the GI lumen, whereas others only receive information from the bloodstream or neighbouring cells and are called closed cells. During embryological development of the GI tract, the enteroendocrine cells have the same origin as absorptive and exocrine secretory cells: progenitor cells in the endodermal epithelium. In the mature intestine, self-regenerative pluripotent stem cells in the crypts differentiate into one of the four lineages of mature epithelial cells: enteroendocrine, goblet, paneth or absorptive (Fig. 7). Differentiation is regulated by specific transcription factors. For example, specific transcription factors, including MATH1 and Neurogenin 3 are necessary for commitment of a Gut Progenitor Cell to the Endocrine Lineage. The presence of other transcription factors is required for terminal differentiation to produce a cell type expressing a specific hormone. For example, Paired Box Protein 4/6 (Pax4/6) is one factor involved in differentiation into GIP cells. THE GASTROINTESTINAL HORMONES A large number of biologically active peptides have been identified in the GI tract. For the current overview, peptides acting primarily as neurotransmitters (neuropeptides), chemokines produced by the immune system and the majority of growth factors will not be considered further. The major hormones and paracrine-acting substances produced, and acting, in the GI tract are presented in Table 2. (Note that Tables 2-4) are for reference). 5 Figure 7. Scheme of the Developmental Pathways of Intestinal Cells (Modified from Fujita et al Pediatric Diabetes. 5 Suppl 2:57-69, 2004) 6 HORMONE Cholecystokinin CELL TYPE CCK GIP K Gastrin G Ghrelin GLP-1 L GLP-2 Histamine Motilin Neurotensin Oxyntomodulin Peptide YY Secretin L ECL Mo N L L S Serotonin (5-HT) Somatostatin EC D MAJOR ACTIONS Stimulation of exocrine pancreatic secretion (protein) and gallbladder contraction; relaxation of Sphincter of Oddi; Satiety Stimulation of insulin biosynthesis and secretion, pancreatic cell mitogenesis and survival; inhibition of acid secretion (?) Stimulation of gastric acid and pepsinogen secretion; Stimulation of pancreatic and gastric mucosal growth Stimulation of food intake Stimulation of insulin biosynthesis and secretion, pancreatic cell mitogenesis and survival; inhibition of glucagon secretion; inhibition of gastric emptying; suppression of food intake Regulation of gastrointestinal growth Stimulation of acid secretion Regulation of the Migrating Myoelectric Complex Regulation of motility Regulation of motility (?); Satiation Gastric Secretion (?); Satiation Stimulation of exocrine pancreatic & hepatic secretion (HCO 3/Water), Regulation of motility Inhibition of exocrine and endocrine secretion and motility Table 2. Major Hormones and Paracrines of the Gastrointestinal Tract (Note that growth factors (e.g. IGF-I, TGF-) and neurotransmitters (e.g. NPY, enkephalins, tachykinins) are not included. GIP = Gastric Inhibitory Polypeptide or Glucose-dependent Insulinotropic Polypeptide; GLP-1 = Glucagon-like peptide-1 7 HORMONE FAMILIES A multitude of bioactive peptides have been discovered, many of which do not have clearly identified functions. Some ‘hormones’ are produced by neurons, in addition to endocrine/paracrine cells. From sequencing and structural studies it has been demonstrated that some hormones can be classified into families, based on homology (Table 4). This can be based on either overall primary structure or regional homology. 1. Overall Primary Structure The PP-fold family of peptides displays sequence similarities of 45-70%. They share a very similar tertiary structure because of homologous residues that are important for stabilization of the 3dimensional fold structure. The main homology within the gastrin family is located in the C-terminal tetrapeptide –Trp-Met-AspPhe-NH2. This region of the molecule is also crucial for biological activity; modification of any residue severely reduces receptor binding and biological activity. There is little similarity in either mRNA or peptide amino acid sequences outside of the common site and immediately adjacent region. Homology among GI peptide hormones, peptide neurotransmitters and growth factors is assumed to reflect their phylogenetic evolution by gene duplication and subsequent mutation (Fig. 9). In some cases there is evidence for a single common ancestor of several peptides with vastly different functions. Receptors of hormones in these families also share regional homology. 2. Regional Homology The major homology of the secretin family resides in the N-terminus (Fig. 8), a region that is critical for biological activity. Removal of the N-terminal 2 amino acids by the enzyme dipeptidyl peptidase IV results in loss, or a significant reduction in, biological activity of these peptides. Secretin Family Gastrin Family Secretin Gastrin Glucagon CCK GLP-1 Cerulein+ GLP-2 Cionin+ GIP VIP PHI GHRH PACAP Insulin Family Insulin IGF-I IGF-II Relaxin PP-fold Family PP PYY NPY Figure 8. Amino acid sequences of the Secretin family Somatostatin Family Somatostatin Corticostatin Motilin Family Motilin Ghrelin Table 4. Gastroenteropancreatic Peptide Families VIP = Vasoactive Intestinal Polypeptide; PHI = Peptide Histidine Isoleucine; GHRH = Growth Hormone Releasing Hormone; PACAP = Pituitary Adenylyl Cyclase-Activating Peptide; IGF-I = Insulin-Like Growth Factor-I; PP = Pancreatic Polypeptide; PYY = Peptide YY; NPY = + Not found in mammals Neuropeptide YY Figure 9. Evolution of the Growth Hormone Releasing Hormone Superfamily 8 GASTROINTESTINAL PEPTIDE HORMONE BIOSYNTHESIS AND SECRETION Introduction As in other cell types, protein synthesis in hormone secreting cells begins with mRNA synthesis in the nucleus and its passage into the cytoplasm. Translation takes place on the ribosomes. The peptide hormone precursor is delivered into the lumen of the rough endoplasmic reticulum, from whence it is transported in transitional vesicles to the Golgi apparatus (Fig. 10). During transport through the cis and trans Golgi, and trans Golgi network (TGN), the hormone precursor is processed and modified in a cell specific manner. Movement between the compartments occurs by budding and fusion of transporting vesicles. Figure 10. Generalized Diagram of the Synthesis and Transport of Protein/Peptide Hormones (Boron, WF & Boulpaep, EL Medical Physiology, 2012) 9 Gene Structure The upstream (5') end of peptide/protein hormone genes generally contains promoter/enhancer regions for regulation of gene transcription. The structural gene consists of variable numbers of introns and exons (Fig. 11) EXON PROMOTER EXON INTRON EXON INTRON INTRON GENE Post-Transcriptional Modification Methyl-G AAAAAAAAA Translation Preprohormone Post-Translational Modification Hormone Figure 11. Outline of Events Involved in the Biosynthesis of a Peptide Hormone. The gene consists of a promoter region and varying numbers of introns and exons. The primary RNA transcript, produced through the action of RNA polymerase, is processed by removal of the introns, followed by capping with 7-methyl guanosine (7Methyl-G) at the 5’ end and addition of a poly-A tail at the 3’ end. Translation on the ribosome results in a peptide precursor, the preprohormone with an N-terminal signal peptide. This is processed to produce mature hormone. 10 Preprohormone Biosynthesis and Processing For the majority of hormones the mRNA codes for a precursor that is considerably longer than the secreted peptide (Fig. 11). This preprohormone contains a signal peptide that is important for directing the peptide chain into the cisternum of the endoplasmic reticulum, where the signal peptide is cleaved off by a peptidase. The prohormone is processed further into the hormone by specific enzymes, that include prohormone convertases (Fig. 12), Preprohormone! Removal of Pre-Peptide Propeptide Cleavage Single Peptide e.g. Motilin, GIP Prohormone! Multiple Peptides e.g. Proglucagon Figure 12. Prohormone Processing Can Result in the Formation of Single or Multiple Hormones Post-Translational Modifications A number of hormone-specific post-translational modifications can occur, including addition of sulphate groups to tyrosine residues. 11 Multiple Hormone Products from a Single Gene 2. Multiple Hormone Products from a Single With many hormone genes, such as that coding for prepromotilin (Fig. 12) there is a single, biologically active hormone produced from the precursor. However, a hormone gene can give rise to more than one hormonal product. There are several examples of prohormones that give rise to more than one biologically active hormone as a result of processing by peptide convertase enzymes. Proglucagon for example is processed to produce mainly GLP-1 and GLP-2 in the intestine and brain neurons, but mainly glucagon in the -cells of the pancreas. Prohormone 1. Multiple Products of a Prohormone with a Single Active Sequence 3. Alternative splicing of RNA transcripts Members of the gastrin family, for example cholecystokinin (Fig. 13), are examples of peptides produced by a single gene that encodes one prohormone from which peptides of different length are cleaved, all of which contain the biologically active region of a single hormone. ProCCK is processed into numerous multiple forms (Fig. 13) whereas Gastrin-17, -34 and-71 are formed from progastrin. Somatostatin-14 and -28 are produced from prosomatostatin, The significance of such complexity is not understood. Following production of the primary RNA transcript, it can sometimes be processed in more than one way, to produce mRNA transcripts that code for alternative peptide products. Examples of this include the secretin gene (Fig. 14) and the gene coding for calcitonin and calcitonin-gene related peptide (CGRP). In the case of secretin, the precursor RNA is processed to produce two mRNA transcripts, one of which codes for a peptide that is processed to form secretin-27 and the other codes for secretin-71. Although different functions for the two peptides have not been established, the larger form exhibits a longer half-life in plasma. Preprohormone Gene! Alternative mRNA Splicing Secretin-27 Figure 13. Alternative Processing of CCK Produces Multiple Products of Different Length Containing the Same C-terminal Biologically Active Region Secretin-71 Figure 14. Alternative mRNA Splicing Results in the Production of Secretin-27 and Secretin-71 12 SECRETION OF GASTROINTESTINAL PEPTIDE HORMONES There are two major pathways by which hormones are secreted in an endocrine or paracrine manner: constitutive and regulated (Fig. 10). Secretory vesicles bud-off from the trans-Golgi network. Small amounts of hormone are transported to the plasma membrane in such vesicles continuously, and in a non-regulated manner. Release of the vesicle contents, which occurs by exocytosis, is termed constitutive secretion. The major pool of peptide hormone is stored until required in secretory granules, which mature from budding vesicles. A specific stimulus to the cell results in fusion of granules with the plasma membrane and release of their contents into the extracellular space. The stimuli that result in secretion are cell type specific. Glucose and GI incretin peptides (GIP, GLP-1) stimulate release of insulin from the -cell. With continuous stimulation granules situated at a distance from the site of release will move along microtubules/microfilaments to the plasma membrane. The overall process is termed stimulus-secretion coupling and this type of secretion is referred to as regulated. Granules are recycled. Following exocytosis hormones either pass through fenestrations (windows) in the capillaries into the bloodstream or act via alternative pathways (e.g. paracrine or autocrine) as described earlier. The secretion of gastrointestinal hormones varies according to the time of day and the frequency and duration of feeding. Figure 15. Histamine Secreted by Enterochromaffin-Like Cells Maintains Basal Gastric Acid Secretion 2. Stimulated Secretion Prior to Food Intake Recent studies indicate that plasma levels of the gastric hormone ghrelin increase progressively during fasting and fall to a nadir within ~1hr. after eating (Fig. 16). Ghrelin has been shown to influence hypothalamic neurons involved in regulating food intake, and evidence is accumulating supporting a role as a trigger for feeding. 1. Basal Secretion It is believed that most, if not all, enteroendocrine cells secrete small amounts of hormone between meals. It is not clear as to whether this ‘basal secretion’ is constitutive or regulated, but in some cases it serves a well-defined function. Secretion of histamine from gastric ECL cells, for example (Fig. 15), is believed to be relatively continuous and to be responsible for maintaining a basal level of acid secretion through paracrine action on parietal cells. In response to feeding, secretion is then potentiated by a number of factors (Fig. 15). Figure 16. Representation of the Relationship Between Ghrelin Secretion and Hunger Several hormones are released prior to food intake as a result of neural signals originating in the brain. This ‘cephalic’ or ‘psychic’ phase of secretion is exhibited by gastrin and CCK. 13 3. Secretion During a Meal Secretion of most GI hormones is stimulated during a meal. Numerous factors can impact on the hormonesecreting cells, including the enteric nervous, endocrine and immune systems (Fig. 17). Some of the endocrine cells also sense the luminal environment and respond to changes in pH or nutrients. The overall secretion rate of a hormone will be determined by the integrated responsiveness to the various stimuli. HCO3- PANCREATIC DUCT CELL! SECRETIN Enteric Nerves Endocrine System Immune System SECRETIN CELL ! H+ LUMEN ! DUODENAL MUCOSA Figure 18. Secretin Secretion in Response to Reduced Intraduodenal pH Nutrients Figure 17. Major Factors Involved in the Regulation of GI Hormone Secretion The specific pathways involved in regulating secretion of the majority of the GI hormones will be discussed later. The secretin, or S-, cell is an example of an open endocrine cell (Fig. 18) that releases its hormone into the bloodstream in response to acid in the lumen of the duodenum. One target organ is the pancreas where it stimulates fluid secretion from duct cells. In contrast, somatostatin and histamine act in a paracrine manner in the stomach: somatostatin inhibiting (Fig. 19) and histamine stimulating acid secretion from parietal cells. Somatostatin can also act in an autocrine manner. Figure 19. Paracrine and Autocrine Actions of Somatostatin 14 4. Interdigestive Secretion of Hormones During the interdigestive period, residual material from previous meals remains in the intestine. Motilin is secreted from the gut (Fig. 20) and appears to modulate the migrating myoelectric complex (MMC) that is responsible of moving the residue down the intestine. It is unclear as to the initiating factor responsible for stimulating motilin, or whether the MMC is a stimulus. Figure 20. Motilin Secretion and the Migrating Myoelectric Complex (MMC) (Physiology 4th Edition. Berne, RM & Levy, MN. Mosby 1998) 15 ACTIONS OF GASTROINTESTINAL HORMONES The various actions of GI hormones will be discussed in later sessions, but it is important to realize that their actions are mediated via specific receptors and signal transduction systems. These receptors may be situated on the cell surface or intracellularly. Molecules acting via cell surface receptors, including peptides, proteins, and small charged molecules such as adrenaline and histamine, are fairly hydrophilic and cannot therefore cross the plasma membrane. Interaction with a cell surface receptor triggers the production of intracellular molecules referred to as second messengers. Intracellular receptors for steroids and the thyroid hormones act as transcription factors and influence gene expression. peptides have binding sites within the transcellular domains. Hormone binding results in activation of a G protein exchange of GTP for GDP and dissociation of from subunits, both of which can be involved in modulating intracellular enzyme activities. This leads to the increase, or decrease, in levels of one or more of several second messengers, including: 1. Cyclic Nucleotides, e.g. Cyclic AMP (Figure 22) 2. Inositol Phosphates and Diacylglycerol (Figure 23) 3. Ca2+ and Calmodulin Figure 22. Receptor-G Protein Mediated Activation of Adenylyl Cyclase CLASSES OF CELL-SURFACE RECEPTORS 1. G Protein-Coupled Seven Transmembrane-Spanning (Heptahelical) Receptors Hormone ²# PEPTIDE HORMONE TM HELICES ! s! GTP # GTP P EXTRACELLULAR SIDE ! s! GDP! The G protein coupled receptors consist of a single polypeptide chain that crosses the membrane seven times. The membrane-spanning regions are helical (heptahelical) (Fig. 21). NH2 Adenylyl Cyclase Receptor ATP cAMP ( ) ATP GDP C C C C R R PKA Protein Phosphorylation Figure 23. Receptor-G Protein Mediated Activation of Phospholipase C g b a Hormone CYTOSOLIC SIDE PIP2 DAG Receptor COOH ²# ! q! Figure 21. Diagram of a Prototypical G Protein-Coupled Receptor Ligand binding of larger polypeptide hormones involves the extracellular N-terminal domain andextracellular loops. Smaller peptides and non- PLC ! q! GDP! IP3 PKC GTP # GTP GDP P Endoplasmic reticulum Ca2+ 16 2. Single Receptors Transmembrane Spanning Polypeptide chains constituting this class of receptors only span the membrane once. Such receptors may consist of a single (EGF) or multiple (insulin/IGF-I) subunits (Fig. 24). However most of the single-chain receptors, such as that for growth hormone, undergo dimerization before or during hormone binding. Growth Hormone Insulin/IGF-I Exterior S a S S S a S S b Tyrosine Kinase Domains b Cytosol P K Kinase P K K Plasma Membrane P K Kinase Figure 24. Representative Examples of Single Transmembrane Spanning Receptors Some of the single transmembrane spanning receptors contain tyrosine kinase domains that are activated on ligand binding (e.g. insulin, EGF) whereas others activate tyrosine kinases bound to specific regions in their intracellular domains. Cascades of kinase phosphorylation can be activated.