How the Turtle Got its Shell
advertisement

Thinking about Turtles and Evolution
Part One: How the Turtle Gets its Shell
This introductory section consists of 5 topics. (1) An initial discussion of “fine tuning” explains
that natural historians have long accepted that natural selection could cause superficial changes
in lineages of organisms. (2) The question of species’ origins, however, is more profound. Here
we explain that, anatomically, turtles are fundamentally different from other vertebrates; the
evolution of turtles is not merely a matter of superficial tinkering with pre-existing body-plans.
(3) In some cases, profound structural changes in plant or animal types are documented by an
extensive fossil record. Such is not the case with turtles, which appear suddenly in the early
Triassic Period. The more we learn about the origin of turtles, the better we will understand the
important concept of evolution and punctuated equilibria. (4) Keys to the mysteries of turtle
evolution should be sought in turtle embryogenesis. The basic question is, how do the shoulder
and hip girdles end up inside of the turtle’s rib-cage? (5) Recent advances in genetics and
developmental biology suggest how such a major anatomical re-arrangement (in the relative
position of ribs and girdles) might result from the redeployment of ancient genetic “construction
programs” to a new purpose.
1. Darwin’s easy sell: fine-tuning by natural selection. General. In the late 1850’s, when
Charles Darwin began to get serious about presenting his views on the origin of species, he did
not have to start from absolute scratch. Most Victorian intellectuals were well aware that
selective breeding could, across multiple generations, lead to discernable differences in separate
lineages of plants or animals. Therefore, Darwin could begin a public lecture by reminding his
audience of how British dairy farmers had developed cows that gave more milk or how pigeon
fanciers had established strains of birds that looked, sounded, and even behaved differently.
Furthermore, many 19th century naturalists were willing to accept that populations of wild
animals could, over generations, also adapt to their particular surroundings. Few thoughtful
biologists were surprised, for example, that European vipers had darker colors in the colder
portions of their range. And these scientists did not in general attribute such coloration to the
direct, causal activity of a divine creator with a particular concern for heat absorption by small,
venomous snakes.
Thinking turtles. Thus biologists operating in a pre-Darwinian paradigm would not have been
surprised to learn that, within a given species of turtle, populations sharing habit with
crocodilians (important turtle predators) have thicker, higher-domed shells than populations
living in croc-free waters. This correlation has been formally demonstrated in North America
and Africa. I suspect it is also true in Asia, Australia, and South America. Evolving a thicker
shell hardly seems surprising. Evolving a shell at all—well, that’s a different matter.
2. The harder question: the origin of species. General. Darwin, of course, was not primarily
interested in showing one more time that selection, natural or otherwise, could fine-tune the fit of
a biotype with its environment. Rather, he wanted to argue that, through descent with heritable
modification, selection could actually result in the origin of radically different biotypes. This
radical idea was hardly even considered by most biologists. Instead, species were thought to be
fundamental elements of the natural world. Sure, biotypes could be manicured a little, by nature
or by human breeders, and perhaps the resulting individuals would fit in a bit better with their
environment. But the basic types, the species, were immutable categories. A cow that gave
more milk was still a cow; a pigeon that rolled was still a pigeon, and a river cooter with a
thicker shell is still a river cooter.
Thinking turtles. Of all the vertebrate animals, none appears so fundamentally different, so
morphologically isolated, as the familiar turtle. Every turtle, of course, sports a suit of protective
armor. But armor in itself does not make the turtle so very different. After all, the number of
armored fishes was once legion. The armored skeletons of dinosaurs populate dioramas in many
of our better museums. Many lizards—and all crocodilians—have their scales underlain by an
armor of dermal bone. The mammalian glyptodonts once carried shells as big as Volkswagens
across the Lowcountry of Pleistocene South Carolina. And living mammals include more than a
dozen armored species of armadillos and pangolins.
However, in two fundamental respects, turtles are different from these other armored creatures.
(1) First, we should recognize that tissues we call “bone” can have two different embryological
origins. The first sort of bone to evolve (in very ancient fish, more than 300 million years ago)
formed by the organization of calcium and phosphorus within the deep layer of the skin called
the dermis. This dermal bone (which persists in human beings as most of the facial bones in our
skulls) entirely comprises the bony shell of every armored vertebrate—except turtles. Turtles,
too, incorporate dermal bone within their shells, but the basic architecture of the carapace (upper
shell) is made of something entirely different: endochondral bone. In vertebrates, endochondral
bone is typically deep bone. It is bone that forms embryologically (or shortly after birth) within
or around cartilage. It is the bone of the vertebrate skeleton—our backbones, our limb bones,
our braincase, our ribs. And how it structures the outside of the turtle is something rather
remarkable. (2) Second, although the turtle’s shell is entirely exterior, it is constructed of deepbody elements. This is obvious when one observes the interior of an empty turtle-carapace: ten
vertebrae are incorporated into this upper shell, and, extending laterally from these vertebrae,
also as parts of the shell, are pairs of ribs (see figure below). In other words, if one looks at an
empty carapace and contemplates the entire turtle, one immediately realizes the deepest mystery
of the turtle’s anatomical evolution: somehow the animal’s limb-girdles are inside the animal’s
rib-cage!
This is no small thing. It is not like a cow that gives more milk or a pigeon that flies in peculiar
patterns. It is a radical variation from the fundamental vertebrate body-plan. Indeed I think that
the evolution of turtles from their shell-less, proto-reptilian ancestors is a critical test-case for
Darwin’s theory on the origin of species. If evolution can explain the origin of turtles, then
everything else should be pretty darn easy.
3. Fossils and the origin of species. General. In some cases an extensive fossil record precisely
chronicles a series of changes leading from one biotype to another. This, however, is not the
general case; indeed, transitions between fossil-bearing geological strata often reflect the
“sudden” appearance of noticeably new organisms as well as the disappearance of older forms.
(Biologists Stephen J. Gould and Niles Ethridge philosophize extensively about this
phenomenon, which they named “punctuated equilibrium.” This topic lies beyond the current
scope of Biology 150, but Gould’s writings are quite interesting and certainly fall within the
intellectual range of a serious college student.)
Thinking turtles. The absence of intermediate forms is particularly evident in the case of turtle
evolution. Approximately 225 million years ago, well before the rise of the dinosaurs, an animal
now called Proganochelys entered the fossil record. We need not concern ourselves with this
varmint’s exact appearance or its poorly understood ecology, but you should understand two
things about beast. First, if you saw the fossils, you would notice the shell and immediately
recognize Proganochelys as a turtle. And, second, this early turtle has no obvious relationship to
any previously living creature. As herpetologist Wayne VanDevender puts it, “Turtles enter the
fossil record with great suddenness, like Athena springing full-blown from the head of Zeus.”
Turtles, then, are not just a quintessential test-case for the explanatory power of Darwinian
theory. Their abrupt appearance, with complete shells, also makes them poster-children for
evolution through punctuated equilibria. For more than a century post-Darwin, the evolutionary
biology of turtles remained essentially unknown, and indeed many mysteries persist today. On
the other hand, new understandings of genetics and embryology are shedding additional light on
the biology of turtles. In fact, turtle-shell formation is a fundamental question of “Evo-Devo,” a
new scientific discipline that is uniting genetics, evolution, and developmental biology.
4. The embryological development of the skeleton. General. In a typical terrestrial-vertebrate
embryo, the pectoral girdle develops as a pair of cartilaginous half-rings that are superficial to
(outside of) the transverse processes of the vertebrae. And, inside the developing girdle, the ribs
extend deep and downward from the vertebrae to form a sort of bone-basket that will support the
viscera. All these deep skeletal elements, initially laid down as cartilage, will, as the embryo
develops, be replaced by bone.
Thinking turtles. The development of the limb-girdles in turtles basically follows the typical
amniote pattern. But the ribs are captured by the spreading pre-skin cells and carried laterally
(not downward), to the outside of the pectoral girdle. In this superficial location they will
establish the skeletal structure of the carapace. Here’s roughly how that works.
All turtles lay eggs, and depending on the kind of turtle, these eggs develop at very different
rates; overall, however, the pattern is pretty much the same across species. At laying, the
embryo within a turtle egg is a hollow mass of cells that has begun to turn inward upon itself to
form what will become three general layers of tissue. Within a few days after laying, the embryo
will have a front end and a back end as well as a top side and a bottom side. This “geography”
of the tiny organism will be mapped by sets of genes that will eventually instruct the embryo to
build particular features in particular places. Among the first recognizable structures to appear
are four limb-buds. These aggregations of cells are located at the front and rear quarters of the
embryo; these will develop according to general patterns recognizable in other tetrapods such as
chickens, mice, and people.
Shortly after the formation of limb-buds, the turtle embryo develops a structure that distinguishes
it from all other vertebrates. This is called the carapacial ridge, and as the name would indicate,
it is associated with the eventual formation of the upper shell. The carapacial ridge first appears
as a slightly thickened line of cells running along each side of the embryo, just above the limb
buds. Tissue-wise, it consists of ectoderm from the embryo’s dorsal side folded atop dermal
mesoderm. In cross-section, the embryo looks like this (From Burke, Amer. Zool., 1991):
Rib-precursors
Expanding edge of the carapacial ridge.
A. Entire embryo in cross-section.
B. Enlargement.
In early cross-sections (see above) the carapacial ridge looks like a structure that might “flow”
down the sides of the turtle embryo, but its actual growth is more to the side, as the entire
embryo flattens. And as this lateral growth accelerates, the cartilaginous rib-precursors
(marked above by yellow arrows) grow laterally along with it. Meanwhile the limb-buds
continue to grow, eventually producing rather conventional front and rear legs for our developing
turtle. And as this occurs, the carapacial ridge, now in apparent control of ribs’ directional
growth, spreads above those legs (and above the shoulder and hip girdles), eventually to form the
turtle’s upper shell.
So that’s how the turtle-shell is formed outside the limb-girdles. The cartilaginous rib-precursors
continue to expand laterally, all the way to what will become the edge of the shell. Then new
cells called osteoblasts migrate from the embryo’s spinal region out and along the rib-precursors,
eventually replacing the softer cartilage with harder bone. Typically this process is more or less
complete by the time the turtle-egg hatches, and visualized by X-ray (to emphasize the bony
structures) the hatchling turtle looks a bit like an elliptical wagon-wheel, with the spine as an
elongated hub and the hardened ribs as the spokes (photo is of hatchling Trachemys scripta, by
Scott Gilbert’s “Team Turtle” at Swarthmore University):
The anatomical emphasis of this essay is the relative position of the turtle’s rib-cage and limbgirdles. You have now learned the basic embryology of this strange contortion, and we shall
presently speculate on how this alteration of vertebrate body-plan might have evolved. First,
however, for completeness’ sake, we’ll briefly consider three other facets of shell-development.
(1) The vertebrae, the ribs, and the margin of the carapace are endochondral bone, “deep bone,”
formed by the replacement of initially cartilaginous tissues (see the photograph immediately
above). The remainder of the carapace—the bone in between the ribs—is dermal bone, laid
down by embryonic skin tissues, typically after the hatching of the little turtle. Herpetologists do
not yet understand exactly how the skin-cells between the ribs “know” that they are supposed to
build bones (while the skin-cells on the tail, legs, and feet, etc., do not); currently most
researchers suspect that the cells within the recently-hardened ribs somehow signal the skin cells
so that they express genes that lead to the construction of the bone. (2) In a healthy, living turtle
the bones of the carapace are covered with other material. In the vast majority of species this
covering consists of laminated plates called scutes. Technically, scutes are scales, and like other
reptile scales they are formed in the epidermis, the outer layer of the skin. Like our fingernails,
turtle scutes are composed of a tough, waterproof, wear-resistant material called keratin. (In case
you’re ever on Jeopardy, you might need to know that human fingernails are made of α-keratin
while most turtle scutes are made of β-keratin, but for our purposes that’s a pretty trivial
difference.) Scutes add strength to a turtle’s shell; because they are pigmented, they also
comprise the patterns that help us distinguish between turtle species. (3) The formation of a
turtle’s plastron, or under-shell, is a topic about which biologists disagree. One theory is that the
bony plastron is formed by ossification of cells whose embryological origin is in the neural crest.
This theory has aroused controversy because cells in the post-cranial neural crest are not
otherwise known to form bone, and cells in the cranial neural crest (which, for example, form
human facial bones) are not otherwise known to migrate posterior to the shoulders. Eventually
we might suggest that a re-mapping of the turtle embryo could cause neural-crest cells to “think”
they should move to the bottom side of the turtle embryo and produce bone. As with the
carapace, bones of the plastron are covered in most turtle species by keratinaceous scutes.
5. Macro-evolutionary changes and tool-kit genes. As explained above, thoughtful preDarwinian biologists were willing to entertain the idea that modest changes in plant or animal
biotypes could result from “selection” (either artificial or natural). Darwin’s principal
difficulties therefore lay in persuading the scientific community that evolution could cause
fundamental alterations of the biotypes themselves. As you probably know, Mr. Darwin’s
recourse was to suggest that small changes, accumulated over vast amounts of time, could result
in radical differences. This remains the basic, generally accepted rationale for macro-evolution.
However, many evolutionists have not felt entirely comfortable with this explanation. Part of the
problem lies in the Darwinian necessity that all forms intermediate between Biotype Alpha and
Biotype Omega should work well—indeed, should work very well, should in general be “fitter”
than their relatives not lying along the trajectory of evolutionary change. In this age of word
processors, we can restate the old monkey-and-typewriter metaphor inflicted upon too many
generations of high school students. The monkey starts with Hamlet, and if she strikes enough
computer keys, then as time stretches towards infinity, her random edits should at some point
produce To Kill a Mockingbird. That sounds believable, given enough random typing. On the
other hand, to assume that an unbroken succession of intermediate drafts would be worth reading
(would exhibit “literary fitness,” to mix academic languages)—well, that would seem to require
a mega-eon of time and a blue bazillion computer-monkeys! But what if a random monkey-
keystroke produced not a letter but an entire word? And what if, by some underlying rule of
grammar, the random change of a word called forth a cascade of other words that would
complete a coherent sentence, and what if other rules of grammar assembled sentences into
paragraphs? Even then a population of computer-monkeys, starting with Hamlet, might not
reproduce Harper Lee’s inspiring novel, but maybe they would eventually write something
interesting.
NOTE: Biology 150 is not by any means a genetics course, but questions about How the Turtle
Gets its Shell are best addressed through the incorporation of evolutionary theory,
developmental biology, and genetics. As background to this discussion, we need to understand,
at least on a metaphorical level, the “grammar” of how the random mutation of one gene can
elicit a cascade of effects that have coherent consequences for a developing organism. In the
long boxed topic presented below, we focus on genetic “switches” to offer a simplistic
explication of that grammar. We hope that you are already familiar with these matters—or that
you can speed-read through our box-topic and move forward to our thinking-turtles section on
toolkit genes.
A. The concept of switches: A simplistic model for understanding genetic principles fundamental to
macro-evolution. Here’s the most basic idea. Many genes do their most important “construction work”
during an organism’s embryological development. And not all genes are turned ON in all parts of the
developing embryo all the time. Now we’ll add a few details.
Most genes function by directing the synthesis of particular proteins. And a gene functions only when it
is turned ON, that is, when an enzyme called RNA-polymerase is able to bind to the gene and make an
RNA copy, or “transcript,” of the gene’s protein-building instructions. In general the RNA-polymerase
can bid to and transcribe a gene only when specific “activator proteins” are bound to a non-coding region
of DNA “upstream” from the gene’s protein-coding instructions. In essence, the binding of an activator
protein will turn the gene “ON,” allowing its transcription. And, on the other hand, the binding of certain
“repressor proteins” will turn the gene OFF, thereby preventing its transcription. The non-coding region
of a gene, to which activator or repressor proteins can bind, may be thought of as a switch by which the
gene’s work is turned ON or OFF. (For a pictorial definition of a genetic switch, see the diagram on the
next page. Also, presently, we shall describe an actual genetic switch.)
You can see that if a gene’s switch-region mutates so that activator and/or repressor proteins cannot
bind to it, then the gene will not be regulated according to the embryo’s original construction-plan.
Imagine now that the mis-regulated gene is involved in creating a certain anatomical structure in the
embryo. The resulting developmental change might be fatal to the embryo or might produce an offspring
that would be poorly fit for long-term survival. But on rare occasions the change in construction-plan
might result in a new structure that would actually be advantageous. (And remember our metaphor. The
re-setting of a switch is not like a one-letter typo in a sentence. It adds or deletes entire words—or
perhaps replaces a sentence as a whole. An increase in literary merit is still unlikely, but it is not a
statistical near-impossibility.) Shortly we shall illustrate this important point by discussing a hypothesis
concerning the reduction of appendages during the evolution of insects.
During embryonic development, numerous genes regulate other genes by making proteins that act
as activators or repressors. Protein from Gene A might act as an activator for Gene B, switching it ON;
protein from Gene B might bind to Genes C and D, switching those OFF, and the absence of repressorproteins from Genes C and D might allow yet another gene to be turned on. Most genes important to
embryonic development have ON-OFF switches. Thus embryonic structures are created by a complex
network of protein-coding genes, their switches, and the activators/repressors that flip those switches at
particular times and in particular anatomical locations. As you read what follows, please keep this idea of
switches in mind. Now here’s a schematic diagram of a switch:
B. Understanding that different switches work at different times in different places. As an
embryonic organism develops, regions become defined in the mass of multiplying cells. This is easy to
understand if you think metaphorically about the embryonic cell-mass as a globe upon which lines of
latitude and longitude are drawn. In an embryo, the “coordinate system” actually defines 4 dimensions:
(1) “anterior-posterior,” (2) “dorsal-ventral,” (3) proximal-distal (“depth” beneath the surface of the cell
mass), and (4) “time” within the schedule of embryonic development. The initial spatial demarcation of
the embryo is defined at various stages in various ways, and the “coordinate system” is increasingly
refined throughout embryonic development. Specific switches are turned ON in some regions and OFF in
others. For example, during the development of an insect embryo, a cascade of switches (…operating on
other switches…) instructs the development of: (1) walking appendages (legs) upon 3 body-segments, (2)
antennae and feeding appendages (mouthparts) on the composite head-segment and (3) no appendages on
the remaining body-segments. Experimentally, biologists can transfer a switch-ON promoter substance to
the cells that will become an insect’s head—and a leg will grow where we would normally expect to see
an antenna.
C. Examining a (partly hypothetical) model for the evolution of insects from ancestors with many
branched appendages. Now let’s consider a simplified scenario for the origin of the Class Hexapoda
(formerly called Class Insecta, the insects). As you follow this scenario, you’ll note that segmentation,
duplication, and multi-functionality are important factors: they allow evolution to tinker with what’s
already there—by means of ON-OFF switches!
Insect legs are unbranched and occur on only 3 body-segments. By contrast, arthropods ancestral
to insects had two-branched appendages on a large number of body-segments. Each appendage typically
had a dual role in locomotion and feeding—however, unbranched, specialized appendages would allow
more efficient walking and would therefore be adaptive, at least for locomotion. Now say that the switch
eliciting the branching of appendages mutates (this would mean that the branching-gene’s activator could
not bind and that the switch would therefore be turned OFF) in one body-region. Nothing super-weird
would happen; instead the organism would have (in the affected region) a set of unbranched appendages
that would be more efficient for walking than the branched appendages of the creature’s ancestors. (Note
the following “small print,” lawyer-type weasel statement: In this caricature-explanation, I am pretending
that the branched appendages mutate from branched to unbranched in one step. In actuality, such would
not be the case; one branch could be deleted over many generations by successive non-expression of a
switch-key protein called distal-less at the termination of that branch.)
Because of the organism’s segmented duplication, the new critter would still have some old-type,
unaffected segments that produce the old-time branched appendages capable of gathering food and of
locomotion—but (because of improved walking-organs in the mutated segments) the locomotive
functions of these unaffected segments would be less important. In these segments, switch-setting
mutations that improve feeding efficiency would therefore be adaptive, even if they undercut the
segments’ locomotive functions. Thus, within the chain of segments that comprise our hypothetical
organism, segments in one region are now somewhat specialized for locomotion, and segments in another
region are now somewhat specialized for feeding. Through further mutations, over time, these
specializations could be fine-tuned, and the organism would become an increasingly efficient walker and
eater. Something like the above scenario really is now accepted as explaining the evolutionary origin of
insects.
D. An Example of a Simple, Prokaryotic Switch. Eukaryotic gene “switches” are more complex than
those of bacteria, but both work in the same general way. So we’ll look at a switch for a bacterial gene as
a simple example. Escherichia coli is a common bacterium (you probably have a few in your body right
now) that survives through the enzymatic metabolism of various sugars. E. coli “prefers” (uh, in so far as
a bacterium can prefer anything…) the simple sugar, glucose. However, if E. coli lives in an environment
with lactose as its energy source, the bacterium will produce the enzyme beta-galactosidase (an enzyme
that is required for the breakdown of lactose). When lactose is absent, E. coli doesn’t “want” to waste the
energy required to produce beta-galactosidase; there’s nothing for the enzyme to digest. As one
contemporary author puts it, “…the neat trick to understand is how the bacterium makes betagalactosidase only when lactose is present. … [T]he production of this enzyme is controlled by a switch
that resides at the beta-galactosidase gene. The switch is off when lactose is absent, but flips on when
lactose is present. There are two key components of the switch, a protein called lac repressor, and the
short stretch of DNA sequence near the beta-galactosidase gene to which the lac repressor protein can
bind. When the repressor protein binds to this DNA sequence, the gene is off (repressed), and no RNA or
protein is made. But when lactose is present, the repressor falls off the DNA, [the promoter-protein can
therefore bind,] and RNA transcription and beta-galactosidase enzyme production occur…[Carroll,
Endless Forms Most Beautiful, 2005].” The action of the LAC Repressor switch is illustrated by the
sketch-model below on the next page.
Thinking turtles. In case you got lost in our box topic about genetic switches, remember that our
current overall topic is Macro-evolutionary changes and tool-kit genes. And now we need to
consider this topic in the context of shell-development. So you Biology 150 students, you need
to hold on through a bit more difficult material; then you’ll be back on more familiar ground.
Perhaps this is the critical part of the Turtle Essay’s “long argument.” At the very beginning you
learned that the origin of the turtle carapace is an understandable problem of macro-evolution,
and you’ve been told that the old-fashioned study of morphogenesis does not explain how/why
the ribs are “captured” and carried outside the limb-girdles by the expansion of the carapacial
ridge. As far as I am aware, the answer to this how/why question is still not entirely known, and
I believe that a few residual unknowns are quite appropriate in a liberal arts course like
Biology150. Most herpetologists think that some answers lie in the re-mapping of the turtle
embryo and in the mobilization for shell-formation of “tool-kit genes” usually associated with
the creation of limb-bones. Here, working backwards from the endpoint (and proceeding from
more certain to less certain), is how I see it:
LAST STAGES OF SHELL DEVELOPMENT: The laterally extended ribs induce intramembranous ossification of dermal bone to complete formation of the upper shell. Presumably
this results from some sort of inter-cellular signaling, by which dermal cells are ordered to make
bone. Pretty much everybody is agreed that this is the case—and that’s not too surprising since
the final ossification of the shell can be followed by X-rays of a growing baby turtle.
MIDDLE STAGES OF SHELL DEVELOPMENT: The lateral migration of rib-precursor
chondroblasts (= cartilage-forming cells) depends on their encounter with the carapacial ridge.
For at least 30 years embryologists have understood that the ribs migrate outside of the limbgirdles (rather than the other way around). Furthermore, recent experiments demonstrate that
when one region of an embryo’s carapacial ridge is surgically removed, developing ribprecursors in that region will alter their developmental trajectories and grow towards the closest
remaining portion of the carapacial ridge. On the other hand, how the carapacial ridge serves as
the critical organizing region is less certain; see below.
FIRST STAGES OF SHELL DEVELOPMENT: The carapacial ridge functions by cooptation of
developmental programs associated in most mammals, birds, and reptiles (including turtles) with
the formation and development of the limb-buds. And now things get shaky, for at this point we
have moved to the frontier of investigations into turtle embryology. The initial hypothesis was
that the carapacial ridge copied, with minor modifications, a pattern well-known in chickembryos, where the development of the apical ectodermal ridge (AER) gave rise directly to limbbuds. As I understand it, the molecular jocks can track this developmental program by observing
the sequential expression of several substances, including, most definitively, Fgf (Fibroblast
growth factor) 10 and then Fgf8. In the turtle’s carapacial ridge, Fgf10 is indeed observed (later,
developmentally, than expected), but Fgf8 is never present. So, that’s a problem for the copythe-AER-pattern hypothesis.
This absence of Fgf8 prompted investigators to posit an alternative hypothesis, that the limb-budlike function of the carapacial ridge works through the cooptation of the canonical Wnt pathway.
OK, oh noble students, “they” didn’t talk this way back when I took cell biology. I don’t
understand quite all of this, I don’t remember much of it, and I certainly won’t test you on it. So
just try to follow the general line of argument. That’s a great academic skill—to follow the
general path when you’re lost on the twists and turns. Good luck with it; we’ll be back on easier
ground soon.
So anyhow, here’s how I understand the deal. Wnt molecules (named for the gene-product
wingless) comprise a set of highly conserved signaling proteins that regulate much inter-cellular
communication during embryological development. Specific Wnt proteins produced by Cell #1
bind to specific receptors on the surface of Cell #2. This binding-signal is transduced across the
cell-membrane to an intracellular protein, β-catenin, that enters Cell #2’s nucleus and initiates
transcription of those genes targeted by the original Wnt signaling proteins.
To test the hypothesis that the carapacial ridge works by coopting the canonical Wnt
pathway, a team of Japanese scientists decided to determine the “location” (in the turtle
embryo’s time and space) at which β-catenin was expressed. These folks found that nuclear
expression of β-catenin did not occur in the embryo’s dorso-lateral region prior to the initiation
of carapacial-ridge development—and that, thereafter, β-catenin was expressed in the carapacial
ridge but not in any surrounding tissues. This finding was interpreted as substantial evidence
that the turtle-embryo’s carapacial ridge could function like a limb-bud because it had co-opted
to that purpose a signaling pathway so ancient that it is shared by insects such as Drosophila, the
mighty fruitfly.
This completes all I know to say about evolutionary changes and tool-kit genes; readers
interested in further information should consult the sundry publications of Shigehiro Kuraku et
al. (Center for Developmental Biology, Kobe, Japan) or the work of Scott Gilbert’s “Team
Turtle” at Swarthmore University. Now I should return to questions about adaptation and the
functions of the turtle’s carapace. Such questions should lead you to a better understanding of
the connections between our classroom discussions and our laboratory exercises this semester.
Part Two: How the Turtle Uses its Shell
1. Evolutionary theory and multi-functionality. General. As early as the time of Darwin,
biologists understood that traits evolved for one purpose were often pressed into additional
duties. Skunks provide a familiar example. These animals belong to an evolutionary lineage
(two families) of mostly nocturnal, mostly solitary mammalian carnivores. Because these critters
seldom encounter other members of their species, they need some way to leave messages about
territorial boundaries, sexual receptivity, etc. To that end, more than 30 million years ago, the
skunk-mustelid lineage evolved perianal glands that secreted persistent, strong-smelling liquids.
Presumably the odors produced by these secretions are not objectionable to conspecifics, but
members of unrelated species generally find them particularly unpleasant. Among proto-skunks
natural selection acted upon these “signaling substances,” increasing the quantity produced,
amplifying the disagreeable aspects of their smell, and modifying the glands’ ducts into an
efficient sprayer-apparatus. Thus, in the chronology of evolution, defense is a secondary
function of skunks’ perianal glands; however, anybody who has experienced the smell, close up
and personal, can attest to its effectiveness.
Thinking turtles. All herpetologists agree that the turtle’s shell evolved as a defensive structure,
protecting the animal’s soft parts against potential predators. Often the defense is effective. As a
child I frequently watched my parents’ dogs attempt without success to chew through the shells
of eastern box turtles (Terrapene carolina). Later, when I was in Hwange National Park
(western Zimbabwe), two adult lions encountered a very large leopard tortoise (Geochelone
pardalis). Obviously hungry, the lions invested considerable time trying to break the shell of the
tortoise; however, the teeth of the great cats merely skittered along the curve of the reptile’s
large, round carapace. Eventually the lions departed with empty stomachs, and, after a respectful
interval, the tortoise crawled on about her business.
Still, although it evolved as a defensive structure, the turtle’s shell serves a number of other
functions as well, and we’ll provide three quick examples. (1) In some cases a rugose shellshape breaks the silhouette of a turtle and helps provide camouflage against a complex
background (see photograph of South American Matamata, Chelus fimbriatus, below).
(2) Tortoises are terrestrial, and many of them inhabit very dry landscapes. In such habitat
water-retention is a constant problem. But tortoise shells are almost perfectly waterproof, and by
reducing the area of porous skin exposed to the sun, their shells protect tortoises from drying out.
(3) Many aquatic turtles spend incredible amounts of time underwater (over 100 days without a
breath of air in hibernating painted turtles, Chrysemys picta). Under such extreme conditions the
turtle’s metabolic rate is greatly reduced, and some species exchange gasses with the water in
which they are immersed. Nevertheless, the unavailability of air results in a tremendous “oxygen
debt”; this the turtle can endure because minerals in its shell-bones buffer its circulatory system
against the buildup of lactic acid that would otherwise prove fatal. I hope you remember this
buffering-function from classes and labs. And I am reasonably sure that you will find much of
the next few pages to be repetitive.
2. The shell and thermoregulation. General. The biochemical reactions that sustain life run
most efficiently within a rather narrow range of temperatures. Therefore, although a few animals
don’t much care how warm or cold they are, most living critters have body-temperatures at
which they prefer to operate. As you know, some creatures like birds and mammals maintain
high and more or less constant body temperatures by eating lots of food and burning calories.
Reptiles (such as turtles) don’t do this; indeed an earlier generation of biologists proclaimed
these animals to be “cold-blooded” and assumed that they accepted the approximate temperature
of their surroundings. Such, of course, is not the case. Most scaly reptiles are ectotherms—that
is, they obtain their heat from the outside—and by behavioral methods, many species under
many conditions can maintain surprisingly precise body temperatures. The “boxed topic” below
reviews the physics of thermoregulation. Such information is not directly relevant to the flow of
this essay, but I thought this would be a decent place to remind you all of what you have learned.
Recapitulation of Thermoregulation in Reptiles
Understanding how reptiles regulate their temperature is like knowing the names of the first five
Old Testament books: such knowledge is not requisite for salvation, but it ought to be! I became
fascinated by thermoregulation during the years when I monitored the body temperature of a
free-ranging canebrake rattlesnake (Crotalus horridus). In summers the snake “preferred” to
stay at about 29oC. She could do this easily in warm weather, adjusting her position between sun
and shade as required. I supposed, on the other hand, that during winter my snake would be cold
all the time. And sure enough, at night or when the weather was cloudy, Ms. Canebrake would
be underground, and she would be winter-cold. When the sun shined, however, things could be
different. I well remember one bright December afternoon when the air temperature was
approximately 5oC. On that cold day the snake had found a slight depression on the ground
surface, directly in the sun and completely out of any wind. The animal had flattened her body
to accept maximum solar radiation—and her body temperature was, as usual, at the preferred
29oC. This rattlesnake was not, in general, a “cold-blooded” animal!
The basic physics of reptilian thermoregulation can be summarized by a simple equation and
diagram:
The change in a reptile’s temperature, Δheat, is the signed sum of heat inputs and heat outputs;
these are conventionally represented by six factors.
(1) The first factor, Met, represents heat produced by the animal’s internal, metabolic processes.
For endotherms, this term is critical, but for most turtles, it is of negligible importance.
(2) The second factor, Qabs, represents absorbed solar radiation, both direct and reflected. As we
shall see, solar radiation is critically important in the thermoregulation of most turtles. This
factor is represented by the equation, Qabs = (S)(A)(vf)(a), where:
• S is solar intensity
– Given sunshine, S is controlled by an animal, as it seeks sun or shade.
• A is surface area of animal
– A is controlled as an animal varies its shape (for example, by pulling legs and
head within the shell or sticking them out).
• vf is view factor
– vf is controlled by an animal’s orientation with respect to the sun (this is less
variable in turtles than among other reptiles; we can design easy ways to measure
it in lab).
• a is absorptivity
– a is largely “built in” by an animal’s color and texture; in some reptiles it is
controlled, in part, by deployment of melanin to/from the skin’s surface, but this
is not very important in most turtles.
(3) The third factor, Con/Evap, represents heat input from the condensation of water vapor and
heat output from the evaporation of water. This factor is not usually important in the
thermoregulation of turtles, except perhaps when aquatic animals first crawl out of the water to
dry and sun.
(4) The fourth factor, Cond, is conduction, and it represents heat-flow between a
thermoregulating animal and some solid object that the animal touches. For many reptiles,
conduction is very important; consider, for example, a desert snake that crawls out to lie on a
warm rock during a cool night. For turtles, typically, conduction is not a critical heat-transfer
process.
(5) The fifth factor, R, represents absorption and emission of infrared radiation. As the
physicists would tell us, any object with temperature > 0oK radiates infrared energy; thus all
reptiles are constantly emitting and absorbing infrared radiation. For larger reptiles, especially at
night, the infrared budget can be quite important, but it is typically swamped by Factor (6),
Convection, in smaller and aquatic animals; in any case this factor has been little studied in
turtles. Infrared heat transfer in a thermoregulating animal is represented by the following
equation, Rnetabs = f {(Te4 – Ts4), ([A][vf]), (EM)}
• Rnetabs represents the net absorption of infrared radiation.
• Te represents the composite surface temperature of objects in the environment of the
thermoregulating animal; Ts represents the surface temperature of the animal itself.
• A and vf are as defined in (2) above.
• EM represents infrared emissivity, how well the surface of the thermoregulating animal
absorbs versus reflects infrared radiation. (This has been very little studied in reptiles,
except desert lizards.)
(6) The final factor, Conv, represents convection, or heat-exchange with a fluid (such as air or
water) in which an animal is immersed. Convection, so important for aquatic turtles, is
represented by the following equation, Cv = f{ (Tf –Ts), (exposure), (CC), (S&BL)}
• Tf is the temperature of the fluid in which the animal is immersed; Ts is the surface
temperature of the animal. Turtles can control Tf in part by selection of microhabitat.
• A thermoregulating animal can control exposure, at least in part, by seeking/avoiding
wind (and water-current); again, control involves selection of microhabitat.
• CC, an animal’s convective coefficient, involves:
– Velocity of fluid flow
– Characteristic dimension (diameter in dimension parallel to airflow)
• S&BL, size and boundary layer, relates to the laminar flow of a fluid around an object.
For instance, because of friction, the air nearest a large object flows more slowly than air
at a greater distance. Thus:
– Larger objects have larger boundary layers (& thus lower convective exposure)
– E.g., a hatchling turtle may “submerge” itself in the boundary layer of a large
basking-rock and therefore be less affected by convective cooling from a chilly
breeze.
Thinking turtles. Herpetologists believe that, when active, many turtles typically like to stay at
about 30oC. And they have many ways to chase that ideal temperature. Terrestrial turtles can
seek sun or shade, to warm up or cool off as necessary. Aquatic turtles, of course, spend a good
bit of time swimming around, but when the water is below their preferred body temperature, they
often crawl out onto sunny logs to bask. Thus, if you watch turtles in the wild, you will often
observe them changing positions within the thermal landscape and adjusting their orientations
with respect to the sun. If, like me, you are particularly interested in reptilian thermal ecology,
you may be tempted to assume that a turtle’s life is largely a matter of thermoregulation. Such,
however, is definitely not the case! Turtles do not live in order to thermoregulate; they
thermoregulate in order to live—in order to feed, in order to avoid enemies, in order to
reproduce, all just a little bit better. Furthermore, a turtle’s requirements for daily living
sometimes actually interfere with the process of efficient thermoregulation. This is a fact that
one needs to consider when one designs a program of field research. And, in another long boxed
topic, I’ll address this issue with respect to the gopher tortoise (Gopherus polyphemus), an
endangered species in our home state of South Carolina.
Here is a bit more review of a PowerPoint that you have already seen. Gophers are Lowcountry
animals that live in well-drained sandy soils, usually dominated by wiregrass, longleaf pine, and
turkey oak. These tortoises are called gophers because, like their mammalian namesakes, they
dig burrows and spend most of their time underground. Constructed by scraping and compaction
of sand, a gopher’s burrow is something of an engineering marvel; it is also vital to gopher
survival because it provides protection from potential enemies and from thermal extremes.
In winter gophers spend > 99% of the time in their burrows. In mid to late spring, they emerge
for an hour or two, every day or so, to feed, court, and breed. And, later, they may remain
occasionally active well into the fall. But I want us to focus for the moment on gopher tortoises
in summer.
Biologists who work summers in South Carolina’s Gopher Tortoise Reserve often brag that
Tillman Sand Ridge is as hot as the suburbs of hell, and indeed afternoon highs do sometimes
exceed 40oC. For this reason we usually assumed that gophers would spend the heat of the day
deep in their burrows (where the temperature is a more reasonable 28o-32o), emerging to feed
only in the early mornings to and late twilight. But such is not the case. Perhaps because
gophers evolved to avoid predators (which would not be active in the noonday sun), these
tortoises come out to feed in the hottest parts of the day. In the summer of 2006, as part of a
state-sponsored research project, we placed recording thermometers and light-meters (“loggers”)
on the shells of two gopher tortoises. A recording of appreciable light indicated that a tortoise
was outside of its burrow—and such expeditions occurred almost exclusively during the heat of
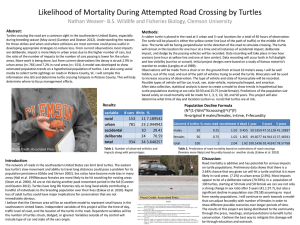
the day. And did those turtle-carapaces get hot! The graph below shows the thermal biography
of a South Carolina gopher between July and October of 2006.
Apparently, the gopher carrying our logger would make brief, daily forays from its burrow out
into the heat of the day. Basically, the animal moved rapidly, eating as much as it could as its
temperature went higher and higher. Then, perhaps just before it over-heated, the gopher would
make a quick run back into the comfort of its burrow.
On the graph above, the horizontal yellow and red lines are probably significant. Many
herpetologists believe that a gopher tortoise would suffer appreciable nerve-tissue damage if its
brain ever reached 40oC; virtually all are agreed that a brain temperature of 45o would be fatal.
But this gopher’s shell (or at least the logger on its shell) approached these presumably lethal
temperatures with considerable frequency. What could be going on?
In the boxed topic above, we indicated that a gopher tortoise’s carapace reaches temperatures
that the animal’s brain could not tolerate, and indeed reptile keepers have frequently noted that in
summer the shell of a large tortoise can become almost too hot to touch. On the other hand, we
have observed that basking turtles often expose their shells to the sun so that absorbed radiation
can warm their bodies. This suggests that perhaps a turtle can “decide” whether or not to make a
thermal exchange between its carapace and its deep-body tissue. An analysis of turtle anatomy
and physiology suggest how this might be done. First, we should recall that air can be an
excellent insulator against heat-transfer, and then we should note the location of a turtle’s lungs,
which are positioned above the viscera and are supported by a ligament attaching them to the
carapace and vertebrae:
In other words, under at least some conditions a turtle’s lungs could insulate the animal’s core
against extreme temperatures experienced by the carapace. Furthermore, such insulation may be
augmented by the turtle’s circulatory anatomy. Like all other living reptiles (except birds and
crocodilians), turtles have hearts that appear, upon gross dissection, to be three-chambered.
Because both atria empty into one ventricle, and because this single ventricle supplies blood to
both pulmonary and systemic circulatory systems, the casual anatomist may assume that freshly
oxygenated blood (from the lungs) mixes freely with oxygen-exhausted blood (from the rest of
the body). But such is not the case, as the following “box topic” will indicate.
Most of us recall from high school biology that endothermic vertebrates such as birds and
mammals have four-chambered hearts. We also learned that the paired ventricles maintain a
complete circulation between pulmonary and systemic circulations. The situation in the turtle is
more complex.
It has been known for several years that turtles can to some degree “choose” the fraction of blood
delivered to the lungs. For example, when an aquatic turtle returns to the surface after a long
dive, it breathes deeply, and it differentially shunts blood to the lungs, so that its oxygen supply
can be rapidly replenished. On the other hand, when that turtle makes another dive, it shunts
blood away from the lungs and toward organs (such as the brain) that particularly need the
oxygen already dissolved in the turtle’s blood. (Please recall that we have attempted to suggest
some of these phenomena in our labs.)
The exact nature of this shunting process remains imperfectly understood and varies somewhat
between turtle species. The timing of the ventricular heart-stroke is involved (see the schematic
below). Also, muscular sphincters on pulmonary arteries can constrict, thereby restricting bloodflow to the lungs. For some details, take a look at the heart-diagram on the following page.
The details of these complex processes are not important for this course. However, in order to
design our field research, we do need to understand the basic idea that turtles can to a substantial
degree regulate blood-flow to various parts of their bodies.
OK, folks, let’s summarize and see where we are in this long document. The title of this section
is “How the Turtle Uses its Shell.” We are hoping that you’ve formulated the idea that a turtle
may use its carapace as a solar collector—and that when the turtle is sufficiently warm (or the
carapace is too hot) the turtle might, through various circulatory mechanisms, isolate the
carapace thermally from the rest of the body.
Part Three: How We Have Been Working to Investigate Ways in which
the Turtle Uses its Shell
All right, Biology 150 students, by this point you should have explicitly recognized connections
between this essay and the conduct of our semester-class. Of course we have not always had a
tremendous amount of success, but such is the nature of scientific research. Anyhow, I have
learned a great deal, and I hope you have too. As the semester matures, I want you to think hard
about the interplay amongst cellular level, organismal level, and ecosystem-level approaches to
the study of biology. That interplay will define the sorts of questions that I’ll try to invent for the
final examination.