WCGME-IRB The Wright Center for Graduate Medical Education
advertisement

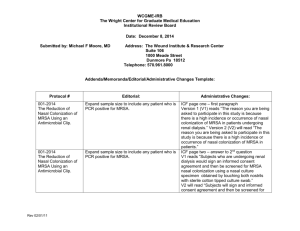
WCGME-IRB The Wright Center for Graduate Medical Education Institutional Review Board Mark V. White, M.D., Chairman To: Robert H. Angeloni, MPA, Co-Chairman Members of the WCGME-IRB From: Mark V. White, M.D., Chairman WCGME-IRB Re: Agenda for the WCGME-IRB meeting on Thursday, January 22, 2015 at 8:00 a.m. in the 2nd floor small Meeting Room, Regional Hospital of Scranton, Scranton, PA 18510. Date: January 7, 2015 Protocols, reports, and items submitted for agenda sections 3, 5, 6 and 7 are available on the WCGME-IRB web page. Click on the “Institutional Review Board” link on The Wright Center web page (www.thewrightcenter.org). Click on the Member Resources link and enter the password. You will need a current version of Adobe Acrobat Reader to view the file. 1. Review of minutes and reports of actions taken: Review of the November 20, 2014 minutes. Minutes are included on the web page and in the meeting documentation packet. 2. Addenda/Memoranda/Editorial/Administrative Changes: 2.1. Scranton Hematology – Oncology Protocol # Editorial: IBCSG 24-02 DSMC recommendations S1207 Everolimus IND safety report N9831 Addendum 20 Changes reflect merger - Alliance CALGB 40101 Update 12 Editorial changes- Protocol termination N0147 Termination of patient follow- up WCGME-IRB Meeting Agenda Page 1 of 9 Administrative Changes: Protocol # Editorial: NSABP B 47 Upcoming closure – reaching accrual goal E 5508 Update 1 – editorial changes PACCT 1 Update 10 – editorial changes Administrative Changes: 2.2 The Wound Institute & Research Center –Dr. M. Moore Protocol # Editorial: 001-2014 The Reduction of Nasal Colonization of MRSA Using an Antimicrobial Clip. Expand sample size to include any patient who is PCR positive for MRSA. 001-2014 The Reduction of Nasal Colonization of MRSA Using an Antimicrobial Clip. Expand sample size to include any patient who is PCR positive for MRSA. 001-2014 The Reduction of Nasal Colonization of MRSA Using an Antimicrobial Clip. 001-2014 The Reduction of Nasal Colonization of MRSA Using an Antimicrobial Clip. Update to reflect current contact. Expand sample to include any patient who is PCR positive for MRSA. WCGME-IRB Meeting Agenda Page 2 of 9 Administrative Changes: ICF page one – first paragraph Version 1 (V1) reads “The reason you are being asked to participate in this study is because there is a high incidence or occurrence of nasal colonization of MRSA in patients undergoing renal dialysis.” Version 2 (V2) will read “The reason you are being asked to participate in this study is because there is a high incidence or occurrence of nasal colonization of MRSA in patients.” ICF page two – answer to 2nd question V1 reads “Subjects who are undergoing renal dialysis would sign an informed consent agreement and then be screened for MRSA nasal colonization using a nasal culture specimen obtained by touching both nostrils with sterile cotton tipped culture swab.” V2 will read “Subjects will sign and informed consent agreement and then be screened for MRSA nasal colonization using a nasal culture specimen obtained by touching both nostrils with sterile cotton tipped culture swab.” ICF page 4 – V1 contact “Dr. Kenneth Rudolph” change to “Dr. Mark V. White” Protocol 2.2 V1 reads “the outpatient dialysis population with a known high incidence of nasal colonization by MRSA would be assessed using polymerase chain reaction (PCR) assays.” V2 will read “the subject would be assessed using polymerase chain reaction (PCR) assays.” Protocol # 001-2014 The Reduction of Nasal Colonization of MRSA Using an Antimicrobial Clip. Editorial: Administrative Changes: Expand sample size to include any patient who is PCR positive for MRSA. Protocol 3.1 V1 reads “ A total of thirty dialysis patients that are PCR positive for MRSA will undergo quantitative cultures to determine the range of colony forming units in asymptomatic colonization.” V2 will read “ A total of thirty subjects that are PCR positive for MRSA will undergo quantitative cultures to determine the range of colony forming units in asymptomatic colonization.” Protocol 3.4 V1 reads “A physician would recruit subjects who are undergoing outpatient renal dialysis. V2 will read “A physician will recruit subjects.” Protocol 6.3 V1 reads “Kenneth H Rudolph, M.D. khrudolph@verizon.net” V2 will read “Mark V White, MD, MPH Chairman, WCGME IRB whitemv@thewrightcenter.org 3. Amendments requiring full board review and approval: None presented for this month. 4. Adverse Drug Reactions – IND Safety Reports: 4.1. Scranton Hematology Oncology Organization and Protocol Number: Millennium C16010 C16040 Patient Identifier: Date: Initial: FU: Adverse Event / Safety Report: 11/4/14 MLN9708 Safety Updates 2013-00863 fu 8-interstitial lung disease, fever, anemia 2014MPI001014 fu 6 adenocarcinoma of colon 2014MPI002402 fu 1 MDS, Pneumonia 2014MPI002682 fu 1- cardiac failure 2014MPI002855 fu 1- acute mesenteric ischemia 2014MPI002902 initial- interstitial lung disease, fever, anemia 2014TJP01414 fu 2- acute bronchitis 2014TJP014698 initial decreased appetite TCI2014A02711 fu 4- angina pectoris 11/5/14 2014MPI001107 fu 1- atrial fibrillation WCGME-IRB Meeting Agenda Page 3 of 9 Organization and Protocol Number: Patient Identifier: Date: Initial: 11/7/14 11/10/14 11/12/14 11/14/14 11/17/14 11/20/14 WCGME-IRB Meeting Agenda Page 4 of 9 FU: Adverse Event / Safety Report: 2014MPI001328 fu 6- seizures 2014MPI002584 fu 2- general physical health deterioration 2014MPI002586 fu 1- acute cholecystitis 2014MPI002841 initial- infection 2014MPI002845 initial- UTI, polyomavirus test positive 2014MPI002853 initial- melena, haematemesis 2014MPI002871 initial- pneumonia 2014MPI000550 fu 3 –seizures 2014MPI002584 fu3- general physical health deterioration 2014MPI002705 fu 2- malaise 2014MPI002853 fu1- melena, haematemesis 2014MPI002907 initial- edema 2014MPI002201 fu 2- influenza, staphylococcus aureus infection 2013-05262 fu 1-cutaneous eruption 2014MPI000166 fu 2- TIA 2014MPI001932 fu 3- tumor lysis syndrome 2014MPI002583 fu 1- febrile neutropenia 2014MPI002662 fu 1- arm phlebitis 2014MPI002916 initial- chest pain 2014MPI002945 initial- pneumonia 2014MPI002950 initial- bilateral pulmonary embolism 2014TJP014698 fu 1- decreased appetite TCI2014A02711 fu 5- angina pectoris 2013MPI000959 fu 1- pulmonary embolism, pneumonia, febrile neutropenia 2013MPI001165 fu 2- pneumonia 2014MPI00267 fu 3 respiratory tract infection, pyrexia 2014MPI002402 fu 2- MDS, pneumonia 2014MPI002621 fu 2 – arrhythemia 2014MPI002855 fu 1 acute mesenteric ischemia 2014MPI002964 initial- femoral neck fracture 2014MPI002965 initial- infectious pneumopathy 2014MPI003009 initial- cardiac arrest 2014MPI002980 initial- pulmonary edema 2014MPI002990 initial- basal cell carcinoma 2014MPI003062 initial- cutaneous eruption 2013MPI001444 initial- febrile Organization and Protocol Number: Patient Identifier: Date: Initial: 11/21/14 11/24/14 11/26/14 12/1/14 12/3/14 12/4/14 WCGME-IRB Meeting Agenda Page 5 of 9 FU: Adverse Event / Safety Report: neutropenia 2013MPI001452 fu 3- gout 2014MPI001891 fu 3- urinary retention 2014MPI002012 fu3- parainfluenza virus 3, febrile illness, confusion 2014MPI002241 fu 2- herpes zoster 2014MPI002574 fu 2- atrial fibrillation 2014MPI002641 fu 1- dehydration 2014MPI002871 fu1 pneumonia 2014MPI002945 fu1 pneumonia 2014MPI003021 initial-heart failure 2014MPI001299 fu 5- malaise 2014MPI001460 fu 1- pneumonia 2014MPI002525 fu 1- acute renal failure, hypoglycemia 2014MPI003100 initial- COPD 2013MPI001261 fu 1 – atrial flutter 2014MPI001825- initial – urosepsis, pyelonephritis, thrombocytopenia, cardiac failure, DVT, hyperthermia 2014MPI002845 fu 1 – UTI 2014TJP014698 fu 2- decreased appetite2013-03958 fu 4- COPD 2013MPI001443 fu 2- Acute renal failure, hypotension 2014MPI001107 fu 2 – atrial fib 2014MPI001318 fu 1- coronary artery thrombosis 2014MPI001349 fu 1- pneumonia 2014MPI002621 fu 3- arrhythmia 2014MPI002980 fu 1- pulmonary edema 2014MPI003075 initial – general physical health deterioration 2014MPI003082 initial- pyrexia 2014MPI003125 initial- myocardial infarction 201302614 fu 2- atrial fib 201305262 fu 2- cutaneous eruption 2014MPI001373 fu 5 – syncope, fall 2014MPI003100 fu 1- COPD 2013MPI000648 fu 2 – hypotension 2013MPI000888 fu 1- C-diff 2014MPI000537 UTI fu2 2014MPI001245 organising pneumonia 2014MPI002845 fu 2 UTI 2014MPI002964 fu 1 femur fracture 2014MPI002980 fu 2 pulmonary edema 2014MPI003119 initial –herpes foster 2014MPI003138 initial – pneumonia 2013-00516 fu 1 Acute renal failure 2014MPI000772 fu 2- MI 2014MPI001107 fu 3 Atrial fibrillation 2014MPI001825 fu 1 urosepsis, DVT, cardiac failure, hyperthermia 2014MPI001976 fu 2 idiopathic Organization and Protocol Number: Patient Identifier: Date: Initial: FU: Adverse Event / Safety Report: thrombocytopenic purpura 2014MPI003151 initial – peripheral atery thrombosis 2014MPI003165 initial -hypercalcemia 2014MPI003166 initial- acute renal failure 2014MPI003170 initial- pain IBCSG 24-02 11/13/14 11/19/14 S1207 11/13/14 11/19/14 12/5/14 11/04/14 E1609 11/18/14 11/5/14 NSABP B 47 11/17/14 Tamoxifen 10002560 depression 10002565 uterine adenomyosis Tamoxifen + Triptorelin 10002562 endometrial polyp 10002572 depression 10002589 uterine fibroids Everolimus PHHO2014FR008155- abdominal pain/ encephalopathy PHHO2014US013821- nodular regenerative hyperplasia- lung infection PHHO2014US013821 fu 1 Ipilimumab 21194865 encephalitis Action letter dated 10/24/14 12/15/14 Trastuzumab 799942- initial – contralateral breast cancer Updated Trastuzumab IB version 12 dated October 2013 E5508 12/3/14 Patient # 55890 expired – metastatic disease E 1609, E3612 12/18/14 MDX-010 #2432573-ISR- Grade 3 encephalopathy, grade 3 renal/urinary disorder: neurogenic bladder E3612,E5103, E5202, E5204, E5508 12/18/14 Bevacizumab # 21498240 FU 1- grade 5 death: NOS E 4805 12/18/14 VEGF-Trap (aflibercept)# 2806788- grade 3 pancreatitis 5. Full Continuing Review: 1o reviewer is: Mark B. White, MD, MPH Chairman, WCGME-IRB 2o reviewer is: Robert Angeloni WCGME-IRB Meeting Agenda Page 6 of 9 5.1.1 Martin Hyzinski, M. D. NSABP: Protocol No.: B 47 “A Randomized Phase III Trial of Adjuvant Therapy Comparing Chemotherapy Alone (Six Cycles of Docetaxel Plus Cyclophosphamide or Four Cycles of Doxorubicin Plus Cyclophosphamide Followed by Weekly Paclitaxel) to Chemotherapy Plus Trastuzumab in Women with Node-Positive or High-Risk Node-Negative HER2-Low Invasive Breast Cancer” was submitted for full continuing review. The study is not permanently closed to enrollment and extension with review in one year is requested. 5.1.2 Martin Hyzinski, M. D. ECOG: Protocol E 3612 “A randomized Phase II Trial of Ipilimumab with or without Bevacizumab in Patients with Unresectable Stage III or Stage IV Melanoma” was submitted for full continuing review. The study is not permanently closed to enrollment and extension with review in one year is requested. 5.1.3 Martin Hyzinski, M. D. SWOG: Protocol S1207 “Phase III Randomized, Placebo-Controlled Clinical Trial Evaluating the Use of Adjuvant Endocrine Therapy +/- One Year of Everolimus in Patients with High-Risk, Hormone Receptor-Positive and HER2/neu Negative Breast Cancer. E^3 Breast Cancer Study- evaluating everolimus with endocrine therapy” was submitted for full continuing review. The study is not permanently closed to enrollment and extension with review in one year is requested. 5.1.4 Sonia Planey, PhD. Protocol TCMC02182013SP “ Validation of APF as Urinary Biomarker for Interstitial Cystitis” was submitted for full continuing review. The study is not permanently closed to enrollment and extension with review in one year is requested. 6. Expedited Continuing Review: 1o reviewer is: Mark B. White, MD, MPH Chairman, WCGME-IRB 2o reviewer is: Robert Angeloni 6.1.1 Martin Hyzinski, M. D. IBCSG: Protocol No.: 24-02 Pharma Analysis. Study Title: “Cognitive Function Substudy ANZ 0701 (Co-SOFT)” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year was requested. 6.1.2. Martin Hyzinski, M. D. NSABP: Protocol No.: B 36 “A Clinical Trial of Adjuvant Therapy Comparing Six Cycles of 5-Fluorouracil, Epirubicin and Cyclophosphamide (FEC) to Four Cycles of Adriamycin and Cyclophosphamide (AC) in Patients With Node-Negative Breast Cancer” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year was requested. 6.1.3 Martin Hyzinski, M. D. CALGB: Protocol No.: C 9344 “Doxorubicin Dose Escalation, With or Without Taxol, as Part of the CA Adjuvant Chemo Regimen for Node Positive Breast Cancer:A Ph.III Intergroup Study” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.4 Martin Hyzinski, M. D. ECOG: Protocol No. E 1496 “Randomized Phase III Study in Low Grade Lymphoma Comparing Maintenance Anti-CD20 Antibody versus Observation Following Induction Therapy” was submitted for expedited continuing review. The Study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.5 Martin Hyzinski, M. D. ECOG: Protocol E2197 “Phase III Study of Adriamycin/Taxotere vs. Adriamycin/Cytoxan in the Adjuvant Treatment of Node Positive and High Risk Node Negative Breast Cancer” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.6 Martin Hyzinski, M. D. ECOG: Protocol No: E2805 “ASSURE: Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.7 Martin Hyzinski, M. D. ECOG: Protocol No.: E 5204 “Intergroup Randomized Phase III Study of Postoperative Oxaliplatin, 5-Fluorouracil and Leucovorin vs Oxaliplatin, 5-Fluorouracil, Leucovorin and Bevacizumab for Patients with Stage II or III Rectal Cancer Receiving Preoperative Chemoradiation” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.8 Martin Hyzinski, M. D. GOG: Protocol No.: GOG 0218 “A Phase III Trial of Carboplatin and Paclitaxel Plus Placebo VersusCarboplatin and Paclitaxel Plus Concurrent Bevacizumab (NSC#704865,IND#7921) Followed by Placebo, Versus Caroboplatin and Paclitaxel Plus Concurrent and Extended Bevacizumab, in Women with Newly Diagnosed, Previously Untreated, Stage III or IV Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. WCGME-IRB Meeting Agenda Page 7 of 9 6.1.9 Martin Hyzinski, M. D. IBCGS: Protocol No. 24-02 “A Phase III Trial Evaluating the Role of Ovarian Function Suppression and the Role of Exemestane as Adjuvant Therapies for Premenopausal Women with Endocrine Responsive Breast Cancer” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.10 Martin Hyzinski, M. D. ECOG: Protocol No.: PACCT- 1 “Program for the Assessment of Clinical Cancer Tests (PACCT-1): Trial Assigning Individualized Options for Treatment: The TAILORx Trial” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.11 Martin Hyzinski, M. D. SWOG: Protocol No.: S0221 “Phase III Trial of Continuous Schedule AC + G vs. Q 2 Week Schedule AC, Followed by Paclitaxel Given Either Every 2 Weeks or Weekly for 12 Weeks as Post-Operative Adjuvant Therapy in Node-Positive or High-Risk Node-Negative Breast Cancer” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year is requested. 6.1.3. William Heim, M.D. Protocol No. ECOG EST 4494: “A Phase III Trial of CHOP versus CHOP and Chimeric Anti-CD20 Monoclonal Antibody (IDEC-C2B8) in Older Patients with Diffuse, Mixed, Diffuse Large Cell and Immunoblastic Large Cell Histology Non-Hodgkin’s Lymphoma” was submitted for expedited continuing review. The study is permanently closed to enrollment but following patients. Extension with review in one year was requested. 6.2 Protocols Terminated/Final Combination Reports 6.2.1 Martin Hyzinski, M. D. CALGB Protocol 40101 “Cyclophosphamide and Doxorubicin (CA x 4 Cycles) Versus Paclitaxel (4 Cycles) as Adjuvant Therapy for Breast Cancer in Women with 0-3 Positive Axillary Lymph Nodes: A Phase III Randomized Study” was submitted as a final report. No further review is necessary. 6.2.2 Martin Hyzinski, M. D. CALGB: Protocol 70604 “A Randomized, Phase III Study of Standard Dosing Versus Longer Dosing Interval of Zoledronic Acid in Metastatic Cancer” was submitted as a final report. No further review is necessary. 6.2.3 Martin Hyzinski, M. D. NSABP: Protocol No.: B 44-I “BETH Study: Treatment of HER2 Positive Breast Cancer With Chemotherapy Plus Trastuzumab vs Chemotherapy Plus Trastuzumab Plus Bevacizumab” was submitted as a final report. No further review is necessary. 6.2.4 Martin Hyzinski, M. D. NCCTG: Protocol N 0147 “A Randomized Phase III Trial of Oxaliplatin (OXAL) Plus 5-Fluorouracil (5-FU)/Leucovorin (CF) with or without Cetuximab (C225) After Curative Resection for Patients with Stage III Colon Cancer” was submitted as a final report. The study is closed to follow-up. No further review is necessary. 6.2.5 William Heim, M. D. ECOG: Protocol E1996 “Phase III Evaluation of EPO With or Without G-CSF versus Supportive Therapy Alone in the Treatment of Myelodysplastic Syndromes” was submitted as a final report. The study is closed to follow-up. No further review is necessary. A separate report indicating death of one of the subjects followed related to progressive disease is on file. 6.3 Expedited New Reviews The following expedited reviews were conducted by the Chairman and/or the Co-Chairman. They are presented for the Board’s information. Complete documentation for these studies is available for inspection by IRB Members in the WCGME-IRB Administrative office. 6.3.1 Dipti Pancholy, M. D. “To study the current trends and best practices in transition of care among hospitalized patients and develop measures to improve them to provide better patient care; A prospective observational study-Phase 2 ” was reviewed and approved by Mark. V. White, M. D. on November 12, 2014. This one year study was approved for one year and must receive continuing review by November 11, 2015 in order to be continued. The study has been assigned the number WCGME11122014DP. 6.3.2 Janet Townsend, M. D .“Assessment of TCMC Graduate Preparedness for Residency Training” was reviewed and approved by Robert H. Angeloni, Co-Chairman on December 9, 2014. This one year study was approved for one year and must receive continuing review by December 8, 2015 in order to be continued. The study has been assigned the number TCMC11202014JT. WCGME-IRB Meeting Agenda Page 8 of 9 6.3.3 Shalane Vitris, ESU Student “Assessing the Utility of a Web Portal in Garnering Interest in the Dual MD/MPH Degree Program: Medical Students’ Perceptions” was reviewed and approved by Robert H. Angeloni, Co-Chairman on December 10, 2014. This one year study was approved for one year and must receive continuing review by December 9, 2015 in order to be continued. The study has been assigned the number ESU09182014SV. 6.3.4 Tiffany Jasulski, Senior MEDENT Specialist, The Wright Center “Emergency Room Care Coordination with the Primary Care for all Asthma Patients and Adults with an Admitting Diagnosis of Chest Pain” was reviewed and approved by Mark. V. White, M. D. on December 23, 2014. This one year study was approved for one year and must receive continuing review by December 22, 2015 in order to be continued. The study has been assigned the number WCGME12152014JT. 6.3.5 Brian Cebulko, PGY-1 Pharmacy Resident-Pharmacist “Prevalence of Early Onset Neonatal Group B Streptococcus Infection Following Standard Intrapartum Vancomycin Prophylaxis” was reviewed and approved by Mark. V. White, M. D. on November 10, 2014. This one year study was approved for one year and must receive continuing review by November 9, 2015 in order to be continued. The study has been assigned the number WU10172014BC. 7. New / Full Review: None submitted for this month 8. Other Business: 9. Dates to Remember: A twelve month running list of WCGME-IRB meetings IRB Meeting Date: 22 January 2015 26 February 2015 26 March 2015 23 April 2015 28 May 2015 25 June 2015 Materials Due By: 29 December, 2014 02 February 2015 02 March 2015 30 March 2015 30 April 2015 1 June 2015 IRB Meeting Date: July – Recess 27 August 2015 24 September 2015 22 October 2015 19 November 2015 December – Recess WCGME-IRB Meeting Agenda Page 9 of 9 Materials Due By: 10 August 2015 03 September 2015 28 September 2015 26 October 2015