Manganese Dioxide Nanostructures as Efficient Oxygen–Evolving
advertisement

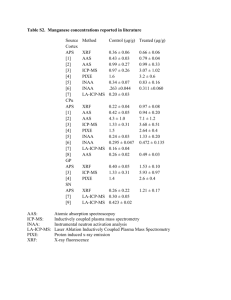
Manganese Dioxide Nanostructures as Efficient Oxygen–Evolving Catalyst in Photosystem II Mostafa Khaksar, Davar M. Boghaei Department of Chemistry, Sharif University of Technology, Azadi Ave., Tehran, Iran dboghaei@sharif.edu Abstract The study of catalytic water oxidation is one of the most active areas of research across many subdisciplines of chemistry from efforts toward developing artificial photosynthetic assemblies to the exploration of nanoscale materials to be used for splitting water. Water oxidation by abundant low-cost materials is of key importance for sustainable energy technologies. An efficient system for water oxidation exists in the water oxidizing complex bound to the proteins of photosystem II in plants, algae and certain bacterias [1-3]. It serves as a model to split water by sunlight. One challenge in this field is the development of artificial model compounds to study oxygen evolution reaction outside the complicated environment of the enzyme. To evolve oxygen efficiently it is necessary first to synthesize what we may call a "super catalyst" for water oxidation [4]. In this study manganese dioxide nanostructures were synthesized via an oxidation-reduction reaction mechanism at mild temperatures, and characterized by FESEM, XRD, XPS, FTIR, TGA and BET. It was found that the morphology of manganese dioxide nanostructures was significantly influenced by the pH of the reaction system. The FESEM images of MnO2 show the large aggregated clusters (Fig.1). Manganese dioxide nanostructures showed high oxygen evolution activity in the presence of cerium(IV) ammonium nitrate. In comparison to the biological paragon the turnover frequencies of catalysts are still low. We identify three common features likely being necessary for catalysis of water oxidation by manganese dioxide nanostructures, which are (i) a mean oxidation state of manganese between +3 and +4, (ii) extensive di-µ-oxido bridging involving coordinatively unsaturated µ2-o(h) bridges between manganese ions, and (iii) high surface area of the manganese dioxide nanostructures (Fig.2). Further studies are required to clarify whether the catalytic performance can be increased by e.g. an optimization of the metal stoichiometry, the structure of the materials at atomic and nanometer scales or the proton management [5]. References [1] M. M. Najafpour, International Journal of Hydrogen Energy, 10 (2012) 8753. [2] M. Wiechen, et al., Dalton Transaction, 1 (2012) 21. [3] S. Petrie, et al., Chemistry-A European Journal, 18 (2008) 5482. [4] J. P. McEvoy, et al., Chemical Reviews, 11 (2006) 4455. [5] J. Yana, et al., Photosynthesis Research, 3 (2007) 289. Figures Figure 1. FESEM images of manganese dioxide nanostructures. Figure 2. BET plot of manganese dioxide nanostructures.