EricksonEsperanzaSyllabusAPchem
advertisement

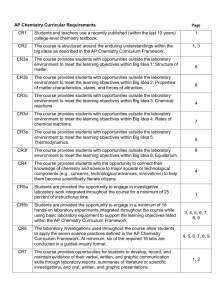
AP Chemistry Syllabus Jocelyn Erickson Nueva Esperanza Academy Curricular Requirements CR1 CR2 Page(s) 1 Students and teachers use a recently published (within the last 10 years) college -level chemistry textbook. The course is structured around the enduring understandings within the big ideas as described in the 1, 3 CR3a AP Chemistry Curriculum Framework. The course provides students with opportunities outside the laboratory environment to meet the 6 CR3b learning objectives within Big Idea Structure of outside matter. the laboratory environment to meet the The course provides students with1:opportunities 6 learning objectives within Big Idea 2: Properties of matter-characteristics, states, and forces of CR3c attraction. The course provides students with opportunities outside the laboratory environment to meet the 4 CR3d learning objectives within Big Idea Chemical reactions. The course provides students with3:opportunities outside the laboratory environment to meet the 7 CR3e learning objectives within Big Idea 4: Rates of chemical reactions. The course provides students with opportunities outside the laboratory environment to meet the 5 learning objectives Bigwith Ideaopportunities 5: Thermodynamics. CR3f The course provides within students outside the laboratory environment to meet the learning objectives within Big Idea 6: Equilibrium. CR4 8 4 The course provides students with the opportunity to connect their knowledge of chemistry and science to major societal or technological components (e.g., concerns, technological advances, help them scientifically literate citizens. laboratory work integrated CR5a innovations) Students aretoprovided thebecome opportunity to engage in investigative CR5b 1 throughout the course for a minimum of 25 percent of instructional time. Students are provided the opportunity to engage in a minimum of 16 hands -on laboratory experiments 3, 4, 5, 6, 7, 8, 9 integrated throughout the course while using basic laboratory equipment to support the learning listedinvestigations within the AP used Chemistry Curriculum Framework. CR6 objectives The laboratory throughout the course allow students to apply the seven science 3, 4, 5, 6, 7, 8, 9 practices defined in the AP Chemistry Curriculum Framework. At minimum, six of the required 16 labs in a guided-inquiry CR7 are Theconducted course provides opportunitiesformat. for students to develop, record, and maintain evidence of their verbal, written, and graphic communication skills through laboratory reports, summaries of literature or scientific investigations, and oral, written, and graphic presentations. Course Description: The purpose of Advanced Placement Chemistry is to provide a college level course in chemistry and to prepare the student to seek credit and/or appropriate placement in college chemistry courses. There is one section of AP chemistry each year with juniors and seniors. All students have had one year of chemistry I prior to taking AP Chemistry. This course meets five times per week for a single 45 minute period and a 90 minute laboratory period. Total class time each week will be 315 minutes, which will include 90 minutes of lab time. Little time is spent on lecture since it is my philosophy that learning is active not passive. Students are engaged in hands -on laboratory work, integrated throughout the course that accounts for more than 25% of the class time. 2 [CR5a] Emphasis is placed on depth of understanding of a topic, rather than breadth of topics. One day per week is spent in study groups using old AP Chemistry Free Response questions/Study Guides for review. Objectives: Students will: 1. Learn the inquiry process through numerous laboratory investigations. 2. Gain an understanding of the six big ideas as articulated in the AP Chemistry Curriculum Framework. [CR2] 3. Apply mathematical and scientific knowledge and skills to solve quantitative, qualitative, spatial, and analytic problems. 4. Apply basic arithmetic, algebraic, and geometric concepts. 5. Formulate strategies for the development and testing of hypotheses. 6. Use basic statistical concepts to draw both inferences and conclusions from data. 7. Identify implications and consequences of drawn conclusions. 8. Use manipulative and technological tools. 9. Measure, compare, order, scale, locate, and code accurately. 10. Do scientific research and report and display the results of this research. 11. Learn to think critically in order to solve problems. Text: -Chemistry Eighth Edition; Zumdahl/Zumdahl Brooks/Cole Cengage Learning Belmont, CA, 2012 ISBN-13:978-0-547-16829-6 [CR1] Lab & Review resources: -Wards AP Chemistry Laboratory Instructions -Wards Laboratory Kits -AP Chemistry Guided-Inquiry Experiments; College Board New York, NY, 2013. -5 steps to a 5, Fifth Edition; Langley & Moore McGraw-Hill; ISBN-13: 978-0071803731 -Previous AP Lab questions Teaching strategies: 1. Summer Assignment - Each student completed a summer assignment that was designed to review chemistry I and identify what students needed to learn before the start of AP Chemistry. The assignment covered chapters 1-5, and parts of chapters 8 and 9. It was designed to include between 5 – 10 questions per topic. Students will be tested on material approximately two weeks into the year. 2. Classes – Every class period is designed to be interactive with the students. The types of classes are lecture, questions and answers, use of the Smart Board, video clips, demonstrations, and group activities. 3. Working Together - During all types of class time, students are encouraged to participate and help in the process. Students are also encouraged to collaborate with each other. It is expected that the students work cooperatively to analyze problems systematically. 4. Homework – Each chapter will have a set of problems that are required for each student to complete. Students will also be required to complete chapter reviews that will include questions from past AP Chemistry test to help prepare them for the test. 5. Quizzes – Every chapter will include one quiz (two for the long chapters). The quizzes will include both multiple choice and free response questions from old AP exams. 6. Tests – Each Unit will end with a multiple-choice test, students will have 90 minutes to complete the test. A comprehensive, standardized semester exam is administered at the end of 1 st semester and a final exam at the end of the year. 7. Labs – All of the laboratory experiments in this course are hands-on. One double period a week will be used for labs. All students will complete at least 16 of the suggested 22 labs. They will work with a lab partner, and when needed, two lab groups will work together. They will follow all safety rules and lab directions to collect, process, manipulate, and graph both quantitative and qualitative data. Students will keep a lab notebook, where they will set up the lab, record all observations, do calculations, error analysis, and write their conclusion. Formal lab reports will be typed after the labs are completed for 6 labs. The back of the lab notebook will have a chemical reference; students will add one of the chemicals used in the lab experiment. Inquiry is emphasized in many of the experiments that students complete. The laboratory work requires students to design, carry out, and analyze data using guided inquiry principles. For all labs, students are required to report the purpose, procedure, all data, data analysis, error analysis, results, and conclusions in a lab report that is submitted for grading. [CR7] All laboratory experiments are intended to be completed in one double period (95 minutes) except the following guided-inquiry labs that require two days of work or two double lab periods: 1. Determination of the Formula of a Compound 2. Finding the Ratio of Moles of Reactants in a Chemical Reaction 3. Progressive Precipitation 4. Relationship Between the Spectrum and Absorbance of Light 5. Conductivity of Solids & Metals 6. Factors that affect reaction rates and determining reaction rates and reaction mechanisms 7. E q u i l i b r i u m P os i t i o n 8. H ydrol ysis of Salt s 9. El ect roch em i cal C el l s All other labs: 10. Determination of Molar Volume of a Gas 11. Standardization of a Solution using a Primary Standard 12.Determination of Concentration by Oxidation-Reduction Titration 13. Determination of Molar Volume of a Gas 14. Determination of Enthalpy Change associated with a Reaction 15. Determination of Electrochemical Series 16. Electroplating 17. Freezing Point Depression to Determine Molar Mass 18. Determination of Molar Mass by Vapor Density 19. Preparation and the Properties of Buffer Solutions 20. Preparation of Aspirin and Analysis of Product Purity 8. Grading System – Student’s grades are based on categories. Total points earned divided by points offered for type of assignment then multiplied by the following percentage: Homework 5%, Classwork 10%, Projects 10%, Lab 25%, Tests/Quizzes 20%, and Midterm/Final 30%. Projects will be an analysis of an old chemical apparatus in quarter 1, a 5-page Research Paper in quarter 2, a poster on a chemist using the Chemical Heritage website for quarter 3, and a group lesson/powerpoint on one of the types of organic compounds for quarter 4. 9. AP review sessions - are held every Tuesday for the school year. Two full AP Practice exams will be taken, one in February and one in April. The students will also take three Multiple Choice practice exams through out the school year. In class review will begin 3 weeks before the exam, where students will complete one free response and 5 multiple choice questions daily and review the topic of the day. 10. AP Exam – all students taking the AP Chemistry class are expected to take the AP Chemistry Exam in May. 11. After the AP exam – We will work on Organic Chemistry. Students will have a combination of lecture, group work, and labs for Organic Chemistry. 12. Technology – Students will use Scientific Calculators in both their class work and laboratory work. Graphs are produced using excel. Students will have access to the internet and computers for work both in and outside of class. Edmodo and the class website will be used by the students and teacher to communicate. 13. Lab Notebook - A laboratory notebook is required for the course. All completed lab reports documenting all lab experiences must be included in the notebook. The notebook is checked every three weeks with a final check at the end of the course. [CR7] 14. Quartly Assingments - This course provides students with the opportunity to connect their knowledge of chemistry and science to major societal or technological components by looking at the concerns, technological advances, and innovations to help them become scientifically literate citizens. Quarter 1 Project: Students will identify older chemistry apparatuses; determine the uses, who discovered it, and the technological advancements in this chemistry apparatuses. Quarter 2 Project: Students will write a 5 page research paper on an everyday object. In the research paper students will identify who invented this object, how the innovation was discovered, what was the purpose of the invention, any new improvements, and the chemistry behind it. Quarter 3 Project: Students will create a poster on a chemist using the Chemical Heritage Society website. They will include background on the chemist, what the discovery was, and how and when it was discovered. Quarter 4 Project: Students will work in small groups to research and present one of the functional groups of Organic Chemistry to their classmates. Pre-Knowledge: Molar Relationships, Electronic Structure, Periodicity, History of Atomic Theory, Intramolecular and Intermolecular Bonding, Molecular Geometry, Gas Laws, Solutions and Colligative Properties, and Nuclear Chemistry Course Outline: [CR2] Chapters in Zumdahl Chemistry AP Chemistry Topic Covered 1 . C hem ical F o un da t io n s None 2 . Atoms, Molecules, and Ions Atomic Theory & Atomic Structure (BI 1 & 2) 3 . Sto i ch i om e t r y Stoichiometry (BI 3) 4 . Solution Stoichiometry & Chemical Analysis Reaction Types & Stoichiometry (BI 3) 5 . Gases Gases (BI 1 & 2) 6 . Thermochemistry Thermodynamics (BI 5) 7 . Atomic Structure and Periodicity Atomic Theory & Atomic Structure (BI 1 & 2) 8 . Bonding -- General Concepts Chemical Bonding (BI 1 & 2) 9 . C ovalent Bo nd in g: Orb ita ls Chemical Bonding (BI 1 & 2) 10. Liquid s and S oli ds Liquids & Solids (BI 1 & 2) 11. Properties of Solutions Solutions (BI 2) 12. Chemica l Kin eti cs Kinetics (BI 4) 13. Chemica l Equ ili brium Equilibrium (BI 6) 1 4 . Acids and Bases Equilibrium (BI 6) 1 5 . Applica t ion s o f Aqu e ou s Equ ilibr ia Equilibrium (BI 6) 1 6 . Spontaneity, Entropy, and Free Energy Thermodynamics (BI 5) 17. Electrochemistry Reaction Types (BI 3) 1 8 . The Nucleus -- A Chemist’s View Nuclear Chemistry 1 9 . The Representative Elements: Groups 1A Through 4A 2 0 . The Representative Elements: Groups 5A Through 8A 22. Organic Chemistry Descriptive Chemistry (BI 2) AP Chemistry Exam Review All Descriptive Chemistry (BI 2) Descriptive Chemistry (BI) refers to Big Ideas. Big Idea 1 – Structure of matter, Big Idea 2 – Properties of matter-characteristics, states and forces of attraction, Big Idea 3 – Chemical reactions, Big Idea 4 – Rates of chemical reactions, Big Idea 5 – Thermodynamics, Big Idea 6 – Equilibrium. Syllabus: Unit 1: (1.0 weeks) A. Chemical Foundations (Chapter 1) a. Measurement b. Significant Figures c. Density d. Matter B. Atoms, moles, and ions (Chapter 2) a. Structure b. Naming C. Stoichiometry (Chapter 3) a. The mole b. Molar Mass c. Percent Composition d. Formula Determination e. Chemical Equations f. Balancing g. Stoichiometric calculations h. Limiting Reagants Problems: Chapter 1: 1, 8, 29-32, 41-42, 47-50, 61-61 Chapter 3: Stoichiometry worksheets 1-3 Activity: LO 3.6: Use data from synthesis or decomposition of a compound to confirm the conservation of matter and the law of definite proportions. The students present problems to the class in which they demonstrate how to find the empirical formula of a compound from data on the percent composition by mass. [CR3c] Review: 5 steps to a 5 chapter 5 & 7 Labs: [CR5b] & [CR6] Determination of the Formula of a Compound (Guided Inquiry) Determination of Molar Volume of a Gas Essential Knowledge: 1.A.2, 1.B.1, 1.D.3, 2.A-2.D, 2.A.3, 2.B.2, 3.A.1, 3.A.2, 3.B.1, Learning Objectives: 1.3, 1.5, 1.16, 2.1, 2.10, 2.13, 3.2, 3.3, 3.5, Unit 2: (2.0 weeks) A. Types of Chemical Reaction & Solution Stoichiometry (Chapter 4) a. Electrolytes b. Composition of Solutions c. Types of Chemical Reactions d. Acid-Base Reactions e. Oxidation-Reduction Reaction f. Balancing Redox Equations Problems: Chapter 4: 15, 23-42, 45-52, 55, 57-58, 69, 70, 79, 83, 87 Review: 5 steps to a 5 chapter 6 Labs: [CR5b] & [CR6] Standardization of a Solution using a Primary Standard Finding the Ratio of Moles of Reactants in a Chemical Reaction (GuidedInquiry) Determination of Concentration by Oxidation-Reduction Titration Progressive Precipitation (Guided-Inquiry) Essential Knowledge: 1.E.2, 3.A.1, 3.A.2, 3.B.3, Learning Objectives: 1.19, 1.20, 3.2, 3.3, 3.4, 3.9, Unit 3: (1.5 weeks) A. Gas Laws (Chapter 5) a. The Gas Laws b. Ideal gas law c. Gas Stoichiometry d. Partial Pressures e. Kinetic molecular theory f. Effusion and Diffusion g. Grahams Law h. Ideal vs. Real Gas behaviors Problems: Chapter 5: 35, 41-43, 45-53, 55, 63-65, 69, 75, 79, 85-87, 103-106, 125 Review: 5 steps to a 5 chapter 8 Labs: [CR5b] & [CR6] Determination of Molar Volume of a Gas Essential Knowledge: 1.E.2, 5.D.2, Learning Objectives: 1.19, 3.10, Unit 4: (2.5 weeks) A. Atomic Structure and Periodicity (Chapter 7) a. Electronic structure and the Periodic table b. Quantum Numbers c. Electron Orbital Notation d. Electron Configuration Notation e. Lewis Dot Notation f. Periodic functions g. Properties of the elements Problems: Chapter 7: 67-69, 79-82, 99, 107, 145, 329-334 Orbital diagrams of elements 1-20 Noble Gas Configurations of elements 1-20 Lewis & Bohr diagrams of elements 1-20 Activity: LO 1.10: Justify with evidence the arrangement of the periodic table and apply periodic properties to chemical reactivity. Students are given several elements pairing them by families or by period and are asked to rationalize the change inelectronegativity of each group based on the electronic structure of the atom [CR3a] Review: 5 steps to a 5 chapter 5 & 10 Labs: [CR5b] & [CR6] Relationship Between the Spectrum and Absorbance of Light (GuidedInquiry) Essential Knowledge: 1.C.1 Learning Objectives: 1.9 Unit 5: (2.5 weeks) A. Bonding: General Concepts (Chapter 8) a. Types of Chemical Bonds b. Electronegativity c. Electron Configurations d. Lewis Structures e. Octet Rule f. Resonance B. Covalent Bonding: Orbitals (Chapter 9) a. Hybridization b. The Molecular Orbital Model Problems: Chapter 8: 15-17, 37, 38, 49-52, 79-83, 85-92, 97, 107, 108, 111-114, 138 Lewis bonding with CO2, CN Chapter 9: 17, 18, 24, 26, 30, 34, 36, 38, 42, 44, 50, 52, 56 Activity: LO 2.21: Use Lewis diagrams and VSEPR to predict the geometry of molecules, identify hybridization, and make predictions about polarity. Students construct balloon models of the arrangement of pairs of electrons around a central atom. They then draw 2D pictures of these arrangements and apply these to predicting the shapes of molecules. [CR3b] Review: 5 steps to a 5 chapter 11 Labs: [CR5b] & [CR6] Model Kits: Looking at the Geometric Shapes of Covalent Bonds Essential Knowledge: 2.D, 2.D.1, 2.D.4, Learning Objectives: 2.22, 2.24, 2.32 Unit 6: (4 weeks) A. Thermochemistry (Chapter 6) a. Enthalpy & Calorimetry b. Hess’s Law c. Standard Enthalpies of Formation d. Bond Enthalpy e. First Law of Thermodynamics f. Present Sources of Energy B. Spontaneity, Entropy, and Free Energy (Chapter 17) a. Spontaneity Process & Entropy b. Second Law of Thermodynamics c. Free Energy d. Standard Fee Energy Change e. Effects of Temperature f. Free Energy and Equilibrium g. Free Energy and Pressure C. Electrochemistry (Chapter 18) a. Balancing Redox Equations b. Galvanic Cells c. Standard Reduction Potentials d. Cell Potential, Electrical Work, and Free Energy e. Voltaic Cells Problems: Chapter 6: 27-30, 43-46, 59-62, 67-74, 77, 78, 81, 82, 89 Chapter 17: 11, 12, 27-30, 33-35, 37, 38, 41-44, 59-61, 63, 64 Chapter 18: 29-32, 37-40, 47, 48, 55 Activity: LO 5.2: Students relate temperature to the motions of particles, either via particulate representations, such as drawings of particles with arrows indicating velocities, and/or via representations of average kinetic energy and distribution of kinetic energies of the particles, such as plots of the Maxwell-Boltzmann distribution. [CR3e] Students are accountable for answering homework questions about particle motions and kinetic energies of a sample at different temperatures while viewing a Podcast. The podcast begins with particulate animations and the narrator interprets the animations to show how kinetic energy distributions can explain the effect of temperature on the rate of a chemical reaction. The questions lead to the interpretation of activation energy on the distribution curve and eventually the refining of collision theory. Review: 5 steps to a 5 chapter 9 & 16 Labs: [CR5b] & [CR6] Determination of Enthalpy Change associated with a Reaction Determination of Electrochemical Series Measurements using Electrochemical Cells and Electroplating (Guided-Inquiry) Electroplating Essential Knowledge: 5.B.3, 5.B.4, Learning Objectives: 5.6, 5.7, Unit 7: (1.5weeks) A. Liquids and solids (Chapter 10) a. Intermolecular Forces b. Liquid State c. Structure and Bonding of Metals d. Molecular Solids e. Ionic Solids f. Vapor Pressure and Changes of State g. Phase Diagrams Problems: Chapter 10: 45-52, 67-74, 79, 80, 87, 89-92 Review: 5 steps to a 5 chapter 12 Labs: [CR5b] & [CR6] Conductivity of Solids & Metals (Guided-Inquiry) Essential Knowledge: 2.A.3, 2.B.2, 2.D, 2.D.1, 2.D.2, Learning Objectives::2.10, 2.13, 2.22, 2.24, 2.28, Unit 8: (1 week and Winter Break) A. Properties of Solutions (Chapter 11) a. Solution concentration units i. (M, N, m, %, ppm, ppb, X) ii. conversions between units b. Factors Affecting Solubility c. Vapor Pressure of Solutions d. Boiling Point Elevation & Freezing Point Depression e. Colligative Properties f. Colloids Problems: Chapter 11: 29-31, 37, 49-52, 56, 63, 64, 66, 69, 70, 72, 83, 84 Review: 5 steps to a 5 chapter 13 Labs: [CR5b] & [CR6] Freezing Point Depression to Determine Molar Mass Determination of Molar Mass by Vapor Density Essential Knowledge: 5.D.2, Learning Objectives: 3.10 Unit 9: (5 weeks) A. Chemical Kinetics (Chapter 12) a. Reaction Rates b. Rate Laws c. Determining the Form of the Rate Law d. Integrated Rate Law e. Reaction Mechanisms f. Catalysis B. Chemical Equilibrium (Chapter 13) a. Equilibrium Constant b. Equilibrium Constant Expression Involving Partial Pressures c. Solving Equilibrium Problems d. Determining the value of K e. Le Chatelier’s Principle Problems: Chapter 12: 27-30, 33-36, 45-50, 55, 56 Chapter 13: 21, 23, 25-32, 39-48, 51-56, 63, 64, 69, 70 Activity: LO 4.8: Translate among reaction energy profile representations, particulate representations, and symbolic representations (chemical equations) of a chemical reaction occurring in the presence and absence of a catalyst. Students create energy diagrams to explain why catalysts and raising the temperature can increase the rate of a chemical reaction. [CR3d] LO 6.1: Given a set of experimental observations regarding physical, chemical, biological, or environmental processes that are reversible, student is able to construct an explanation that connects the observations to the reversibility of the underlying chemical reactions or processes. Students view the NO2/N2O4 Equilibrium simulation available on the General Equilibria Animations Index page at Iowa State University and verbally report and discuss their answers to teacher supplied questions regarding the number of reactant and product molecules present at a particular point in the equilibrium process, the breaking and forming of bonds during the process, and how the reactant and product molecules are changing in order to illustrate the dynamic nature of equilibrium. [CR3f] Review: 5 steps to a 5 chapter 14 & 15 Labs: [CR5b] & [CR6] E q u i l i b r i u m P os i t i on ( G u i d e d - In q u i r y) Factors that affect reaction rates and determining reaction rates and reaction mechanisms (Guided-Inquiry) Essential Knowledge: 4.A.1, 4.A.3, 4.B.2, 4.D.2, Learning Objectives: 4.1, 4.2, 4.4, 4.5, 4.9, Unit 10: (5 weeks) A. Acids and Bases (Chapter 14) a. pH Scale b. Calculating the pH of Strong Acids c. Calculating the pH of Weak Acids d. Bases e. Acid-Base Properties of Salts f. Solving Acid-Base Problems g. pH, Kw, Ka, Kb B. Acid-Base Equilibria (Chapter 15) a. Buffered Solutions b. Buffering Capacity c. Titrations and pH Curves d. Acid-Base Indicators C. Solubility and Complex Ion Equilibria (Chapter 16) a. Solubility Equilibria and the Solubility Product b. Precipitation and Qualitative Analysis c. Ksp d. Concentration of Ions Problems: Chapter 14: 33, 34, 43-45, 47-53, 55, 56, 61, 62, 69, 71-74, 87-90, 93-96, 115, 116, 125, 126 Chapter 15: 27-31, 33, 35, 36, 53, 55, 56, 61, 63-66 Chapter 16: 21-28, 35-40, 47-51, 65, Review: 5 steps to a 5 chapter 6 Labs: [CR5b] & [CR6] Preparation and the Properties of Buffer Solutions Preparation of Aspirin and Analysis of Product Purity H ydrol ysis of Salts (Guided -Inquir y) Essential Knowledge: 3.B.1, 6.B.1, 6.C.1, 1.E.2, 6.C.2, Learning Objectives: 3.5, 6.9, 6.11, 6.12, 6.13, 6.18, 6.20, AP Exam Review (2 – 3 weeks) A. Emphasize writing net ionic equations, knowing the solubility rules, solving equilibrium problems, and reviewing AP Released Exams. B. Each day will consist of one free response, 5 multiple choice, and a review of one of the topics from the year Unit 11: (2 weeks) A. Organic Chemistry: Students will have a comprehensive look at the six main functional groups in Organic Chemistry Labs: [CR5b] & [CR6] Synthesis, Purification, & Analysis of an Organic Compound, Banana Ester, Silly Putty