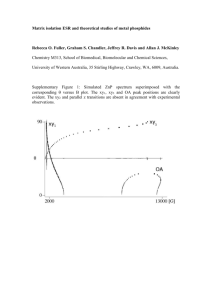
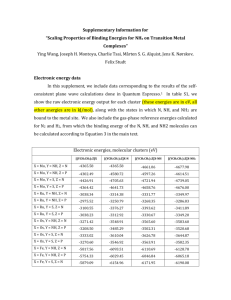
Suplementary A14.07.0074
advertisement

Electronic Supplementary Material Nonorthogonal Orbital Based N-body Reduced Density Matrices and Their Applications to Valence Bond Theory. III. Second-Order Perturbation Theory Using Valence Bond Self-Consist Field Function as Reference Zhenhua Chen, Xun Chen, Fuming Ying, Junjing Gu, Huaiyu Zhang and Wei Wu* The State Key Laboratory of Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005, China. E-mail: weiwu@xmu.edu.cn, Fax: (86)592-2183047 * To whom correspondence should be addressed. 1 Table of contents Table S1. Spectroscopic constants for some diatomic molecules by VBSCF and icVBPT2 methods with OEO. Table S2. The icVBPT2 total energies of H2 at various distances. Table S3. The icVBPT2 total energies of N2 at various distances. Table S4. The icVBPT2 total energies of O2 at various distances. Table S5. The icVBPT2 total energies of F2 at various distances. Table S6. The icVBPT2 total energies of D-A reaction of butadiene and ethylene. 2 Table S2. Spectroscopic constants for some diatomic molecules by VBSCF and icVBPT2 methods with OEO. ωe (cm-1) re (a0) Molecule H2 NVB 3 N2 17 O2 175 12 105 F2 H2 3 3 N2 17 De (eV) O2 175 12 105 F2 H2 3 3 N2 17 O2 175 12 F2 105 3 VBSCF 1.427 2.117 2.119 2.321 2.322 2.768 4206 2353 2342 1567 1567 783.0 4.135 8.246 8.333 3.574 3.614 0.714 icVBPT2 1.408 2.119 2.122 2.320 2.319 2.692 4371 2358 2354 1588 1712 906.3 4.612 8.546 8.636 4.711 4.761 1.508 3 Table S2. The icVBPT2 total energies (E, in a.u.) of H2 at various distances, (r, in a0). r HAO OEO 1.347375 -1.168305 -1.168408 1.404067 -1.169023 -1.169123 1.460759 -1.168552 -1.168651 188.972688 -0.999642 -0.999642 4 Table S3. The icVBPT2 total energies (E, in a.u.) of N2 at various distances, (r, in a0). HAO OEO r NVB = 17 NVB = 175 NVB = 17 NVB = 175 2.05 -109.136460 -109.139818 -109.138996 -109.141995 2.10 -109.139669 -109.143365 -109.142500 -109.145713 2.15 -109.138992 -109.143046 -109.142104 -109.145514 50.00 -108.828715 -108.828715 -108.828715 -108.828715 5 Table S4. The icVBPT2 total energies (E, in a.u.) of O2 at various distances, (r, in a0). HAO OEO r NVB = 12 NVB = 105 NVB = 12 NVB = 105 2.25 -149.863159 -149.865685 -149.869801 -149.871335 2.30 -149.865201 -149.867699 -149.871629 -149.873441 2.35 -149.865200 -149.867655 -149.871443 -149.873208 100.00 -149.698649 -149.698649 -149.698649 -149.698649 6 Table S5. The icVBPT2 total energies (E, in a.u.) of F2 at various distances, (r, in a0). r HAO OEO 2.679633 -199.274789 -199.276918 2.736325 -199.275794 -199.277645 2.793016 -199.275814 -199.277393 188.972688 -199.222254 -199.222254 7 Table S6. The icVBPT2 total energies (in a.u.) of D-A reaction of butadiene and ethylene. Orbital LFO OEO NVBa Reactant Transition state Product 5 -233.7469354 -233.6805364 -233.7699336 17 -233.7469505 -233.7078866 -233.8099284 175 -233.7479637 -233.7143718 -233.8136158 5 -233.7473106 -233.7080892 -233.8124686 17 -233.7473119 -233.711077 -233.8128245 175 -233.7483275 -233.7138082 -233.8140334 a. The number of VB structures involved in the VBSCF wave function. 8